Pericytes contribute to pulmonary vascular remodeling via HIF2α signaling
- PMID: 38243138
- PMCID: PMC10897382
- DOI: 10.1038/s44319-023-00054-w
Pericytes contribute to pulmonary vascular remodeling via HIF2α signaling
Abstract
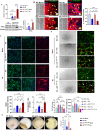
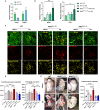
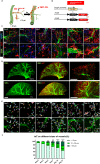
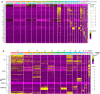
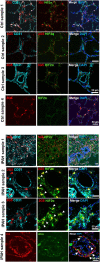
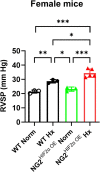
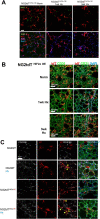
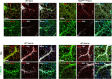
Vascular remodeling is the process of structural alteration and cell rearrangement of blood vessels in response to injury and is the cause of many of the world's most afflicted cardiovascular conditions, including pulmonary arterial hypertension (PAH). Many studies have focused on the effects of vascular endothelial cells and smooth muscle cells (SMCs) during vascular remodeling, but pericytes, an indispensable cell population residing largely in capillaries, are ignored in this maladaptive process. Here, we report that hypoxia-inducible factor 2α (HIF2α) expression is increased in the lung tissues of PAH patients, and HIF2α overexpressed pericytes result in greater contractility and an impaired endothelial-pericyte interaction. Using single-cell RNAseq and hypoxia-induced pulmonary hypertension (PH) models, we show that HIF2α is a major molecular regulator for the transformation of pericytes into SMC-like cells. Pericyte-selective HIF2α overexpression in mice exacerbates PH and right ventricular hypertrophy. Temporal cellular lineage tracing shows that HIF2α overexpressing reporter NG2+ cells (pericyte-selective) relocate from capillaries to arterioles and co-express SMA. This novel insight into the crucial role of NG2+ pericytes in pulmonary vascular remodeling via HIF2α signaling suggests a potential drug target for PH.
Keywords: HIF2α; Pericyte; Pulmonary Hypertension; Vascular Remodeling.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–54. - PubMed
-
- Bordenave J, Thuillet R, Tu L, Phan C, Cumont A, Marsol C, Huertas A, Savale L, Hibert M, Galzi JL, Bonnet D, Humbert M, Frossard N, Guignabert C. Neutralization of CXCL12 attenuates established pulmonary hypertension in rats. Cardiovasc Res. 2020;116:686–697. - PubMed
MeSH terms
Grants and funding
- R01 HL138473/HL/NHLBI NIH HHS/United States
- R01 HL145676/HL/NHLBI NIH HHS/United States
- P30 AR070253/AR/NIAMS NIH HHS/United States
- 13POST17120040/AHA/American Heart Association-American Stroke Association/United States
- R01 HL139664/HL/NHLBI NIH HHS/United States
- I01 BX005628/BX/BLRD VA/United States
- P01 HL152961/HL/NHLBI NIH HHS/United States
- R01 HL172449/HL/NHLBI NIH HHS/United States
- R01 HL150583/HL/NHLBI NIH HHS/United States
- R01 HL158714/HL/NHLBI NIH HHS/United States
- R01 HL150106/HL/NHLBI NIH HHS/United States
- R01 HL134776/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

