Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance
- PMID: 38243280
- PMCID: PMC10797874
- DOI: 10.1186/s12943-024-01932-0
Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance
Abstract
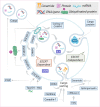
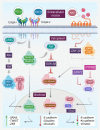
The production and release of tumor-derived small extracellular vesicles (TDSEVs) from cancerous cells play a pivotal role in the propagation of cancer, through genetic and biological communication with healthy cells. TDSEVs are known to orchestrate the invasion-metastasis cascade via diverse pathways. Regulation of early metastasis processes, pre-metastatic niche formation, immune system regulation, angiogenesis initiation, extracellular matrix (ECM) remodeling, immune modulation, and epithelial-mesenchymal transition (EMT) are among the pathways regulated by TDSEVs. MicroRNAs (miRs) carried within TDSEVs play a pivotal role as a double-edged sword and can either promote metastasis or inhibit cancer progression. TDSEVs can serve as excellent markers for early detection of tumors, and tumor metastases. From a therapeutic point of view, the risk of cancer metastasis may be reduced by limiting the production of TDSEVs from tumor cells. On the other hand, TDSEVs represent a promising approach for in vivo delivery of therapeutic cargo to tumor cells. The present review article discusses the recent developments and the current views of TDSEVs in the field of cancer research and clinical applications.
Keywords: Biomarkers; Cancer; Epithelial-mesenchymal transition; Metastasis; MicroRNA; Oncogenic transformation; Tumor microenvironment; Tumor-derived small extracellular vesicles.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

