Faecalibacterium prausnitzii as a potential Antiatherosclerotic microbe
- PMID: 38243314
- PMCID: PMC10797727
- DOI: 10.1186/s12964-023-01464-y
Faecalibacterium prausnitzii as a potential Antiatherosclerotic microbe
Abstract
Background: The gut microbiota plays a crucial role in coronary artery disease (CAD) development, but limited attention has been given to the role of the microbiota in preventing this disease. This study aimed to identify key biomarkers using metagenomics and untargeted metabolomics and verify their associations with atherosclerosis.
Methods: A total of 371 participants, including individuals with various CAD types and CAD-free controls, were enrolled. Subsequently, significant markers were identified in the stool samples through gut metagenomic sequencing and untargeted metabolomics. In vivo and in vitro experiments were performed to investigate the mechanisms underlying the association between these markers and atherosclerosis.
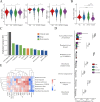
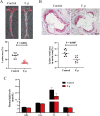
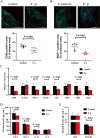
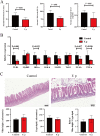
Results: Faecal omics sequencing revealed that individuals with a substantial presence of Faecalibacterium prausnitzii had the lowest incidence of CAD across diverse CAD groups and control subjects. A random forest model confirmed the significant relationship between F. prausnitzii and CAD incidence. Notably, F. prausnitzii emerged as a robust, independent CAD predictor. Furthermore, our findings indicated the potential of the gut microbiota and gut metabolites to predict CAD occurrence and progression, potentially impacting amino acid and vitamin metabolism. F. prausnitzii mitigated inflammation and exhibited an antiatherosclerotic effect on ApoE-/- mice after gavage. This effect was attributed to reduced intestinal LPS synthesis and reinforced mechanical and mucosal barriers, leading to decreased plasma LPS levels and an antiatherosclerotic outcome.
Conclusions: Sequencing of the samples revealed a previously unknown link between specific gut microbiota and atherosclerosis. Treatment with F. prausnitzii may help prevent CAD by inhibiting atherosclerosis.
Keywords: Coronary artery disease; Faecalibacterium prausnitzii; Gut microbiota; Lipopolysaccharide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on Cardiovascular Health and Diseases Burden in China: an Updated Summary of 2020. Chinese Circulation Journal. 2021;36(6) Serial No.276
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

