Gut microbiome for predicting immune checkpoint blockade-associated adverse events
- PMID: 38243343
- PMCID: PMC10799412
- DOI: 10.1186/s13073-024-01285-9
Gut microbiome for predicting immune checkpoint blockade-associated adverse events
Abstract
Background: The impact of the gut microbiome on the initiation and intensity of immune-related adverse events (irAEs) prompted by immune checkpoint inhibitors (ICIs) is widely acknowledged. Nevertheless, there is inconsistency in the gut microbial associations with irAEs reported across various studies.
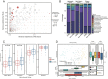
Methods: We performed a comprehensive analysis leveraging a dataset that included published microbiome data (n = 317) and in-house generated data from 16S rRNA and shotgun metagenome samples of irAEs (n = 115). We utilized a machine learning-based approach, specifically the Random Forest (RF) algorithm, to construct a microbiome-based classifier capable of distinguishing between non-irAEs and irAEs. Additionally, we conducted a comprehensive analysis, integrating transcriptome and metagenome profiling, to explore potential underlying mechanisms.
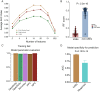
Results: We identified specific microbial species capable of distinguishing between patients experiencing irAEs and non-irAEs. The RF classifier, developed using 14 microbial features, demonstrated robust discriminatory power between non-irAEs and irAEs (AUC = 0.88). Moreover, the predictive score from our classifier exhibited significant discriminative capability for identifying non-irAEs in two independent cohorts. Our functional analysis revealed that the altered microbiome in non-irAEs was characterized by an increased menaquinone biosynthesis, accompanied by elevated expression of rate-limiting enzymes menH and menC. Targeted metabolomics analysis further highlighted a notably higher abundance of menaquinone in the serum of patients who did not develop irAEs compared to the irAEs group.
Conclusions: Our study underscores the potential of microbial biomarkers for predicting the onset of irAEs and highlights menaquinone, a metabolite derived from the microbiome community, as a possible selective therapeutic agent for modulating the occurrence of irAEs.
Keywords: Gut microbiome; Immune checkpoint inhibitors; Immune-related adverse events; Programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1).
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

