Oxygen glucose deprivation-pretreated astrocyte-derived exosomes attenuates intracerebral hemorrhage (ICH)-induced BBB disruption through miR-27a-3p /ARHGAP25/Wnt/β-catenin axis
- PMID: 38243347
- PMCID: PMC10799414
- DOI: 10.1186/s12987-024-00510-2
Oxygen glucose deprivation-pretreated astrocyte-derived exosomes attenuates intracerebral hemorrhage (ICH)-induced BBB disruption through miR-27a-3p /ARHGAP25/Wnt/β-catenin axis
Abstract
Background: Blood brain barrier (BBB) breakdown is one of the key mechanisms of secondary brain injury following intracerebral hemorrhage (ICH). Astrocytes interact with endothelial and regulate BBB integrity via paracrine signaling factors. More and more studies reveal astrocyte-derived extracellular vesicles (ADEVs) as an important way of intercellular communication. However, the role of ADEV in BBB integrity after ICH remains unclear.
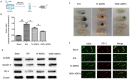
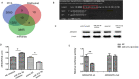
Methods: ADEVs were obtained from astrocytes with or without oxygen and glucose deprivation (OGD) pre-stimulation and the role of ADEVs in ICH was investigated using ICH mice model and ICH cell model. The potential regulatory effect of ADEVs on endothelial barrier integrity was identified by TEER, western blot and immunofluorescence in vitro. In vivo, functional evaluation, Evans-blue leakage and tight junction proteins (TJPs) expression were analyzed. MiRNA sequencing revealed that microRNA-27a-3p (miR-27a-3p) was differentially expressed miRNA in the EVs from OGD-pretreated astrocytes compared with normal control. The regulatory mechanism of miR-27a-3p was assessed using Luciferase assay, RT-PCR, western blot and immunofluorescence.
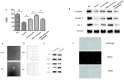
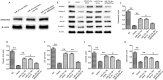
Results: OGD-activated astrocytes reduced hemin-induced endothelial hyper-permeability through secreting EVs. OGD-activated ADEVs alleviated BBB dysfunction after ICH in vivo and in vitro. MicroRNA microarray analysis indicated that miR-27a-3p is a major component that was highly expressed miRNA in OGD pretreated-ADEVs. OGD-ADEVs mitigated BBB injury through transferring miR-27a-3p into bEnd.3 cells and regulating ARHGAP25/Wnt/β-catenin pathway.
Conclusion: Taken together, these findings firstly revealed that miR-27a-3p, as one of the main components of OGD-pretreated ADEVs, attenuated BBB destruction and improved neurological deficits following ICH by regulating endothelial ARHGAP25/Wnt/β-catenin axis. OGD-ADEVs might be a novel strategy for the treatment of ICH. this study implicates that EVs from OGD pre-stimulated astrocytes.
Keywords: Blood brain barrier; Intracerebral hemorrhage; OGD pretreated astrocyte-derived exosome; miR-27a-3p.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11.J Biol Chem. 2018 Dec 28;293(52):20041-20050. doi: 10.1074/jbc.RA118.001858. Epub 2018 Oct 18. J Biol Chem. 2018. PMID: 30337368 Free PMC article.
-
miR-27a-3p regulates expression of intercellular junctions at the brain endothelium and controls the endothelial barrier permeability.PLoS One. 2022 Jan 13;17(1):e0262152. doi: 10.1371/journal.pone.0262152. eCollection 2022. PLoS One. 2022. PMID: 35025943 Free PMC article.
-
miR-666-3p Mediates the Protective Effects of Mesenchymal Stem Cell-derived Exosomes Against Oxygen-glucose Deprivation and Reoxygenation- induced Cell Injury in Brain Microvascular Endothelial Cells via Mitogen-activated Protein Kinase Pathway.Curr Neurovasc Res. 2021;18(1):20-77. doi: 10.2174/1567202618666210319152534. Curr Neurovasc Res. 2021. PMID: 33745435
-
Extracellular Vesicles Derived From Neural Stem Cells, Astrocytes, and Microglia as Therapeutics for Easing TBI-Induced Brain Dysfunction.Stem Cells Transl Med. 2023 Mar 17;12(3):140-153. doi: 10.1093/stcltm/szad004. Stem Cells Transl Med. 2023. PMID: 36847078 Free PMC article. Review.
-
The Potential Role of Exosomes in Communication Between Astrocytes and Endothelial Cells.Int J Mol Sci. 2025 May 14;26(10):4676. doi: 10.3390/ijms26104676. Int J Mol Sci. 2025. PMID: 40429819 Free PMC article. Review.
Cited by
-
The role of potential oxidative biomarkers in the prognosis of intracerebral hemorrhage and the exploration antioxidants as possible preventive and treatment options.Front Mol Biosci. 2025 Feb 4;12:1541230. doi: 10.3389/fmolb.2025.1541230. eCollection 2025. Front Mol Biosci. 2025. PMID: 39967652 Free PMC article. Review.
-
Edaravone dexborneol provides neuroprotective benefits by suppressing ferroptosis in experimental intracerebral hemorrhage.Sci Rep. 2025 May 13;15(1):16595. doi: 10.1038/s41598-025-99187-2. Sci Rep. 2025. PMID: 40360664 Free PMC article.
-
Reactive astrocyte-derived exosomes enhance intracranial lymphatic drainage in mice after intracranial hemorrhage.Fluids Barriers CNS. 2025 Apr 14;22(1):37. doi: 10.1186/s12987-025-00651-y. Fluids Barriers CNS. 2025. PMID: 40229887 Free PMC article.
-
Exosomal MicroRNA: an Effective Strategy for the Treatment of Intracerebral Hemorrhage.Mol Neurobiol. 2025 Aug;62(8):9966-9979. doi: 10.1007/s12035-025-04886-6. Epub 2025 Apr 2. Mol Neurobiol. 2025. PMID: 40175714 Review.
-
Sphk1/S1P pathway promotes blood-brain barrier breakdown after intracerebral hemorrhage through inducing Nlrp3-mediated endothelial cell pyroptosis.Cell Death Dis. 2024 Dec 23;15(12):926. doi: 10.1038/s41419-024-07310-4. Cell Death Dis. 2024. PMID: 39715736 Free PMC article.
References
-
- Al-Shahi Salman R, Frantzias J, Lee RJ, Lyden PD, Battey TWK, Ayres AM, Goldstein JN, Mayer SA, Steiner T, Wang X, Arima H, Hasegawa H, Oishi M, Godoy DA, Masotti L, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Jang DK, Davalos A, Castillo J, Yao X, Claassen J, Volbers B, Kazui S, Okada Y, Fujimoto S, Toyoda K, Li Q, Khoury J, Delgado P, Sabín J, Hernández-Guillamon M, Prats-Sánchez L, Cai C, Kate MP, McCourt R, Venkatasubramanian C, Diringer MN, Ikeda Y, Worthmann H, Ziai WC, d'Esterre CD, Aviv RI, Raab P, Murai Y, Zazulia AR, Butcher KS, Seyedsaadat SM, Grotta JC, Martí-Fàbregas J, Montaner J, Broderick J, Yamamoto H, Staykov D, Connolly ES, Selim M, Leira R, Moon BH, Demchuk AM, Di Napoli M, Fujii Y, Anderson CS, Rosand J. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17:885–894. doi: 10.1016/S1474-4422(18)30253-9. - DOI - PMC - PubMed
-
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis, The Lancet. Neurology. 2010;9:167–176. - PubMed
-
- Wu X, Luo J, Liu H, Cui W, Guo K, Zhao L, Bai H, Guo W, Guo H, Feng D, Qu Y. Recombinant adiponectin peptide ameliorates brain injury following intracerebral hemorrhage by suppressing astrocyte-derived inflammation via the inhibition of Drp1-mediated mitochondrial fission. Transl Stroke Res. 2020;11:924–939. doi: 10.1007/s12975-019-00768-x. - DOI - PubMed
-
- Keep RF, Andjelkovic AV, Xiang J, Stamatovic SM, Antonetti DA, Hua Y, Xi G. Brain endothelial cell junctions after cerebral hemorrhage: changes, mechanisms and therapeutic targets. J Cerebral Blood Flow Metab Off J Int Soc Cerebral Blood Flow Metab. 2018;38:1255–1275. doi: 10.1177/0271678X18774666. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

