A simple and efficient in planta transformation method based on the active regeneration capacity of plants
- PMID: 38243598
- PMCID: PMC11009361
- DOI: 10.1016/j.xplc.2024.100822
A simple and efficient in planta transformation method based on the active regeneration capacity of plants
Abstract
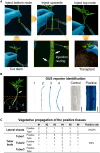
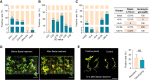
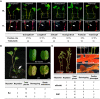
Plant genetic transformation strategies serve as essential tools for the genetic engineering and advanced molecular breeding of plants. However, the complicated operational protocols and low efficiency of current transformation strategies restrict the genetic modification of most plant species. This paper describes the development of the regenerative activity-dependent in planta injection delivery (RAPID) method based on the active regeneration capacity of plants. In this method, Agrobacterium tumefaciens is delivered to plant meristems via injection to induce transfected nascent tissues. Stable transgenic plants can be obtained by subsequent vegetative propagation of the positive nascent tissues. The method was successfully used for transformation of plants with strong regeneration capacity, including different genotypes of sweet potato (Ipomoea batatas), potato (Solanum tuberosum), and bayhops (Ipomoea pes-caprae). Compared with traditional transformation methods, RAPID has a much higher transformation efficiency and shorter duration, and it does not require tissue culture procedures. The RAPID method therefore overcomes the limitations of traditional methods to enable rapid in planta transformation and can be potentially applied to a wide range of plant species that are capable of active regeneration.
Keywords: RAPID; active regeneration; bayhops; plant genetic transformation; potato; sweet potato.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Unlocking regeneration potential: harnessing morphogenic regulators and small peptides for enhanced plant engineering.Plant J. 2025 Jan;121(2):e17193. doi: 10.1111/tpj.17193. Epub 2024 Dec 10. Plant J. 2025. PMID: 39658544 Free PMC article. Review.
-
Efficient embryogenic suspension culturing and rapid transformation of a range of elite genotypes of sweet potato (Ipomoea batatas [L.] Lam.).Plant Sci. 2011 Dec;181(6):701-11. doi: 10.1016/j.plantsci.2011.01.005. Epub 2011 Jan 19. Plant Sci. 2011. PMID: 21958713
-
Sweet Potato [Ipomoea batatas (L.) Lam].Methods Mol Biol. 2006;344:37-44. doi: 10.1385/1-59745-131-2:37. Methods Mol Biol. 2006. PMID: 17033049
-
Agrobacterium-Mediated Transformation of Solanum tuberosum L., Potato.Methods Mol Biol. 2019;1864:203-223. doi: 10.1007/978-1-4939-8778-8_15. Methods Mol Biol. 2019. PMID: 30415339
-
Transformation of medicinal plants using Agrobacterium tumefaciens.Postepy Hig Med Dosw (Online). 2016 Dec 20;70(0):1220-1228. Postepy Hig Med Dosw (Online). 2016. PMID: 28026825 Review.
Cited by
-
Crop genome editing through tissue-culture-independent transformation methods.Front Genome Ed. 2024 Dec 5;6:1490295. doi: 10.3389/fgeed.2024.1490295. eCollection 2024. Front Genome Ed. 2024. PMID: 39703881 Free PMC article. Review.
-
Plant genetic transformation: achievements, current status and future prospects.Plant Biotechnol J. 2025 Jun;23(6):2034-2058. doi: 10.1111/pbi.70028. Epub 2025 Mar 7. Plant Biotechnol J. 2025. PMID: 40052992 Free PMC article. Review.
-
Highly Efficient Regeneration of Bombax ceiba via De Novo Organogenesis from Hypocotyl and Bud Explants.Plants (Basel). 2025 Jul 2;14(13):2033. doi: 10.3390/plants14132033. Plants (Basel). 2025. PMID: 40648042 Free PMC article.
-
Advancements in delivery strategies and non-tissue culture regeneration systems for plant genetic transformation.Adv Biotechnol (Singap). 2024 Sep 26;2(4):34. doi: 10.1007/s44307-024-00041-9. Adv Biotechnol (Singap). 2024. PMID: 39883316 Free PMC article. Review.
-
Unlocking regeneration potential: harnessing morphogenic regulators and small peptides for enhanced plant engineering.Plant J. 2025 Jan;121(2):e17193. doi: 10.1111/tpj.17193. Epub 2024 Dec 10. Plant J. 2025. PMID: 39658544 Free PMC article. Review.
References
-
- Bahramnejad B., Naji M., Bose R., Jha S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol. Adv. 2019;37 - PubMed
-
- Bailey R. ThoughtCo; 2020. Types of Vegetative Propagation.https://www.thoughtco.com/vegetative-propagation-4138604
-
- Bar M., Ori N. Leaf development and morphogenesis. Development. 2014;141:4219–4230. - PubMed
-
- Belehu T., Hammes P.S., Robbertse P.J. The origin and structure of adventitious roots in sweet potato (Ipomoea batatas) Aust. J. Bot. 2004;52:551–558.
MeSH terms
LinkOut - more resources
Full Text Sources

