Safety and effectiveness of abatacept in juvenile idiopathic arthritis: results from the PRINTO/PRCSG registry
- PMID: 38243722
- PMCID: PMC11381685
- DOI: 10.1093/rheumatology/keae025
Safety and effectiveness of abatacept in juvenile idiopathic arthritis: results from the PRINTO/PRCSG registry
Abstract
Objective: The aim of this study was to report the interim 5-year safety and effectiveness of abatacept in patients with JIA in the PRINTO/PRCSG registry.
Methods: The Abatacept JIA Registry (NCT01357668) is an ongoing observational study of children with JIA receiving abatacept; enrolment started in January 2013. Clinical sites enrolled patients with JIA starting or currently receiving abatacept. Eligible patients were assessed for safety (primary end point) and effectiveness over 10 years. Effectiveness was measured by clinical 10-joint Juvenile Arthritis Disease Activity Score (cJADAS10) in patients with JIA over 5 years. As-observed analysis is presented according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
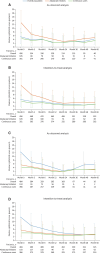
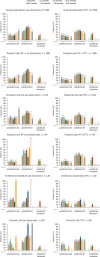
Results: As of 31 March 2020, 587 patients were enrolled; 569 are included in this analysis (including 134 new users) with 1214.6 patient-years of safety data available. Over 5 years, the incidence rate (IR) per 100 patient-years of follow-up of serious adverse events was 5.52 (95% CI: 4.27, 7.01) and of events of special interest was 3.62 (95% CI: 2.63, 4.86), with 18 serious infections [IR 1.48 (95% CI: 0.88, 2.34)]. As early as month 3, 55.9% of patients achieved cJADAS10 low disease activity and inactive disease (20.3%, 72/354 and 35.6%, 126/354, respectively), sustained over 5 years. Disease activity measures improvement over 5 years across JIA categories.
Conclusion: Abatacept was well tolerated in patients with JIA, with no new safety signals identified and with well-controlled disease activity, including some patients achieving inactive disease or remission.
Trial registration: Clinicaltrials.gov, NCT01357668.
Keywords: DMARDs; adolescent rheumatology; biologic therapies; juvenile idiopathic arthritis; paediatric/juvenile rheumatology.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. - PubMed
-
- Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet 2011;377:2138–49. - PubMed
-
- Ringold S, Angeles‐Han ST, Beukelman T et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non‐systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res (Hoboken) 2019;71:717–34. - PMC - PubMed
-
- Lovell DJ, Ruperto N, Giannini EH, Martini A. Advances from clinical trials in juvenile idiopathic arthritis. Nat Rev Rheumatol 2013;9:557–63. - PubMed
-
- Ruperto N, Martini A. Current and future perspectives in the management of juvenile idiopathic arthritis. Lancet Child Adolesc Health 2018;2:360–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

