Two-year efficacy and safety of relugolix combination therapy in women with endometriosis-associated pain: SPIRIT open-label extension study
- PMID: 38243752
- PMCID: PMC10905503
- DOI: 10.1093/humrep/dead263
Two-year efficacy and safety of relugolix combination therapy in women with endometriosis-associated pain: SPIRIT open-label extension study
Abstract
Study question: What is the efficacy and safety of long-term treatment (up to 2 years) with relugolix combination therapy (CT) in women with moderate to severe endometriosis-associated pain?
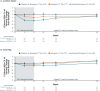
Summary answer: For up to 2 years, treatment with relugolix CT improved menstrual and non-menstrual pain, dyspareunia, and function in women with endometriosis; after an initial decline of <1%, the mean bone mineral density (BMD) remained stable with continued treatment.
What is known already: Endometriosis is a chronic condition characterized by symptoms of dysmenorrhea, non-menstrual pelvic pain (NMPP), and dyspareunia, which have a substantial impact on the lives of affected women, their partners, and families. SPIRIT 1 and 2 were phase 3, randomized, double-blind, placebo-controlled studies of once-daily relugolix CT (relugolix 40 mg, oestradiol 1 mg, norethisterone acetate 0.5 mg) in premenopausal women (age 18-50 years) with endometriosis and moderate-to-severe dysmenorrhea and NMPP. These trials demonstrated a significant improvement of dysmenorrhea, NMPP, and dyspareunia in women treated with relugolix CT, with minimal decline (<1%) in BMD versus placebo at 24 weeks.
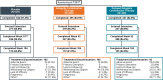
Study design, size, duration: Patients participating in this open-label, single-arm, long-term extension (LTE) study of the 24-week SPIRIT pivotal studies (SPIRIT 1 and 2) received up to an additional 80 weeks of once-daily oral relugolix CT treatment between May 2018 and January 2023.
Participants/materials, setting, methods: Premenopausal women with confirmed endometriosis and moderate to severe dysmenorrhea and NMPP who completed the 24-week pivotal studies (SPIRIT 1 and 2 trials; Giudice et al., 2022) and who met all entry criteria were eligible to enrol. Two-year results were analysed by treatment group based on original randomization in pivotal studies: relugolix CT, delayed relugolix CT (relugolix 40 mg monotherapy for 12 weeks, followed by relugolix CT), or placebo→relugolix CT (placebo for 24 weeks followed by relugolix CT). The primary endpoints of the LTE study were the proportion of dysmenorrhea and NMPP responders at Week 52 and Week 104/end-of-treatment (EOT). A responder was a participant who achieved a predefined, clinically meaningful reduction from baseline in Numerical Rating Scale (NRS) scores (0 = no pain, 10 = worst pain imaginable) for the specific pain type with no increase in analgesic use. The predefined clinically meaningful threshold for dysmenorrhea was 2.8 points and for NMPP was 2.1 points. Secondary efficacy endpoints included change from baseline in Endometriosis Health Profile-30 (EHP-30) pain domain scores, a measure of the effects of endometriosis-associated pain on daily activities (function), NRS scores for dysmenorrhea, NMPP, dyspareunia, and overall pelvic pain, and analgesic/opioid use. Safety endpoints included adverse events and changes in BMD.
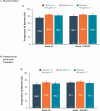
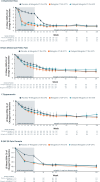
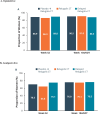
Main results and the role of chance: Of 1261 randomized patients, 1044 completed the pivotal studies, 802 enrolled in the LTE, 681 completed 52 weeks of treatment, and 501 completed 104 weeks of treatment. Demographics and baseline characteristics of the extension population were consistent with those of the original randomized population. Among patients randomized to relugolix CT at pivotal study baseline who continued in the LTE (N = 277), sustained improvements in endometriosis-associated pain were demonstrated through 104 weeks. The proportion of responders at Week 104/EOT for dysmenorrhea and NMPP was 84.8% and 75.8%, respectively. Decreases in dyspareunia and improvement in function assessed by EHP-30 pain domain were also sustained over 2 years. At Week 104/EOT, 91% of patients were opioid-free and 75% of patients were analgesic-free. Relugolix CT over 104 weeks was well tolerated with a safety profile consistent with that observed over the first 24 weeks. After initial least squares mean BMD loss <1% at Week 24, BMD plateaued at Week 36 and was sustained for the duration of 104 weeks of treatment. Efficacy and safety results were generally consistent in women in the placebo→relugolix CT and delayed relugolix CT groups.
Limitations, reasons for caution: The study was conducted as an open-label study without a control group over the 80 weeks of the extension period. Of the 802 patients who were enrolled in this LTE study, 681 patients (84.9%) and 501 patients (62.5%) of patients completed 52 and 104 weeks of treatment, respectively. In addition, there currently are no comparative data to other hormonal medications. Finally, a third (37.4%) of the study population terminated participation early.
Wider implications of the findings: In conclusion, relugolix CT offers an additional option to help address an important unmet clinical need for effective, safe, and well-tolerated medical treatments for endometriosis that can be used longer-term, reducing the need for opioids and improving quality of life. The findings from this study may help support the care of women with endometriosis seeking longer-term effective medical management of their symptoms.
Study funding/competing interest(s): This study was funded by Myovant Sciences GmbH (now Sumitomo Pharma Switzerland GmbH). C.M.B. reports fees from Myovant, grants from Bayer Healthcare, fees from ObsEva, and Chair of ESHRE Endometriosis Guideline Group (all funds went to the University of Oxford); N.P.J. reports personal fees from Myovant Sciences, during the conduct of the study, personal fees from Guerbet, personal fees from Organon, personal fees from Roche Diagnostics; S.A.-S. reports personal fees from Myovant Sciences, personal fees from Bayer, personal fees from Abbvie, personal fees from UpToDate; J.S.P., and R.B.W. are employees and shareholders of Myovant Sciences; J.C.A.F. and S.J.I. are shareholders of Myovant Sciences (but at time of publicaion are no longer employess of Myovant Sciences); M.S.A. and K.W. have no conflicts to declare; V.M. is a consultant to Myovant; L.C.G. reports personal fees from Myovant Sciences, Inc and Bayer. The authors did not receive compensation for manuscript writing, review, and revision.
Trial registration number: NCT03654274.
Keywords: dysmenorrhea; dyspareunia; endometriosis; long-term treatment; non-menstrual pelvic pain; pain; relugolix.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
C.M.B. reports fees from Myovant, grants from Bayer Healthcare, fees from ObsEva all of which went to the University of Oxford, and Chair of ESHRE Endometriosis Guideline Group; N.P.J. reports personal fees from Myovant Sciences, during the conduct of the study, personal fees from Guerbet, personal fees from Abbott, personal fees from Roche Diagnostics; S.A.-S. reports personal fees from Myovant Sciences, personal fees from Bayer, personal fees from Organon, personal fees from UpToDate; J.C.A.F., S.J.I., J.S.P., and R.B.W. are employees and shareholders of Myovant Sciences; J.C.A.F. and S.J.I. are shareholders of Myovant Sciences (but at time of publicaion are no longer employess of Myovant Sciences); M.S.A. and Dr Dynowski, and K.W. have no conflicts to declare; V.M. is a consultant to Myovant; L.C.G. reports personal fees from Myovant Sciences and Bayer. The authors did not receive compensation for manuscript writing, review, and revision.
Figures
References
-
- Barbieri RL. Hormone treatment of endometriosis: the oestrogen threshold hypothesis. Am J Obstet Gynecol 1992;166:740–745. - PubMed
-
- Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ. et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015;163:461–464. - PubMed
-
- Cramer DW, Barbieri RL, Fraer AR, Harlow BL.. Determinants of early follicular phase gonadotrophin and estradiol concentrations in women of late reproductive age. Hum Reprod 2002;17:221–227. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

