LINC MIR503HG Controls SC-β Cell Differentiation and Insulin Production by Targeting CDH1 and HES1
- PMID: 38243869
- PMCID: PMC10987150
- DOI: 10.1002/advs.202305631
LINC MIR503HG Controls SC-β Cell Differentiation and Insulin Production by Targeting CDH1 and HES1
Abstract
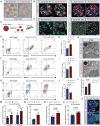
Stem cell-derived pancreatic progenitors (SC-PPs), as an unlimited source of SC-derived β (SC-β) cells, offers a robust tool for diabetes treatment in stem cell-based transplantation, disease modeling, and drug screening. Whereas, PDX1+/NKX6.1+ PPs enhances the subsequent endocrine lineage specification and gives rise to glucose-responsive SC-β cells in vivo and in vitro. To identify the regulators that promote induction efficiency and cellular function maturation, single-cell RNA-sequencing is performed to decipher the transcriptional landscape during PPs differentiation. The comprehensive evaluation of functionality demonstrated that manipulating LINC MIR503HG using CRISPR in PP cell fate decision can improve insulin synthesis and secretion in mature SC-β cells, without effects on liver lineage specification. Importantly, transplantation of MIR503HG-/- SC-β cells in recipients significantly restored blood glucose homeostasis, accompanied by serum C-peptide release and an increase in body weight. Mechanistically, by releasing CtBP1 occupying the CDH1 and HES1 promoters, the decrease in MIR503HG expression levels provided an excellent extracellular niche and appropriate Notch signaling activation for PPs following differentiation. Furthermore, this exhibited higher crucial transcription factors and mature epithelial markers in CDH1High expressed clusters. Altogether, these findings highlighted MIR503HG as an essential and exclusive PP cell fate specification regulator with promising therapeutic potential for patients with diabetes.
Keywords: differentiation; human pluripotent stem cells; lncRNA; pancreatic progenitors; scRNA‐seq.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Magliano D. J., Boyko E. J., in IDF Diabetes Atlas, Brussels: 2023.
-
- a) Hogrebe N. J., Maxwell K. G., Augsornworawat P., Millman J. R., Nat. Protoc. 2021, 16, 4109; - PMC - PubMed
- b) Rezania A., Bruin J. E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T., Yang Y. H., Johnson J. D., Kieffer T. J., Nat. Biotechnol. 2014, 32, 1121; - PubMed
- c) Hogrebe N. J., Augsornworawat P., Maxwell K. G., Velazco‐Cruz L., Millman J. R., Nat. Biotechnol. 2020, 38, 460; - PMC - PubMed
- d) Pagliuca F. W., Millman J. R., Gurtler M., Segel M., Van Dervort A., Ryu J. H., Peterson Q. P., Greiner D., Melton D. A., Cell 2014, 159, 428; - PMC - PubMed
- e) Russ H. A., Parent A. V., Ringler J. J., Hennings T. G., Nair G. G., Shveygert M., Guo T., Puri S., Haataja L., Cirulli V., Blelloch R., Szot G. L., Arvan P., Hebrok M., EMBO J. 2015, 34, 1759; - PMC - PubMed
- f) Zhou Q., Melton D. A., Nature 2018, 557, 351; - PMC - PubMed
- g) Mahaddalkar P. U., Scheibner K., Pfluger S., Ansarullah, Sterr M., Beckenbauer J., Irmler M., Beckers J., Knobel S., Lickert H., Nat. Biotechnol. 2020, 38, 1061. - PubMed
-
- a) Ramzy A., Belmonte P. J., Braam M. J. S., Ida S., Wilts E. M., Levings M. K., Rezania A., Kieffer T. J., Endocr Rev 2023, 44, 222; - PubMed
- b) Yoshihara E., O'Connor C., Gasser E., Wei Z., Oh T. G., Tseng T. W., Wang D., Cayabyab F., Dai Y., Yu R. T., Liddle C., Atkins A. R., Downes M., Evans R. M., Nature 2020, 586, 606. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
