Temporal change of DNA methylation subclasses between matched newly diagnosed and recurrent glioblastoma
- PMID: 38244080
- PMCID: PMC10799798
- DOI: 10.1007/s00401-023-02677-8
Temporal change of DNA methylation subclasses between matched newly diagnosed and recurrent glioblastoma
Abstract
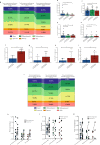
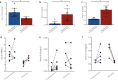
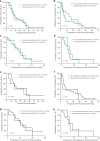
The longitudinal transition of phenotypes is pivotal in glioblastoma treatment resistance and DNA methylation emerged as an important tool for classifying glioblastoma phenotypes. We aimed to characterize DNA methylation subclass heterogeneity during progression and assess its clinical impact. Matched tissues from 47 glioblastoma patients were subjected to DNA methylation profiling, including CpG-site alterations, tissue and serum deconvolution, mass spectrometry, and immunoassay. Effects of clinical characteristics on temporal changes and outcomes were studied. Among 47 patients, 8 (17.0%) had non-matching classifications at recurrence. In the remaining 39 cases, 28.2% showed dominant DNA methylation subclass transitions, with 72.7% being a mesenchymal subclass. In general, glioblastomas with a subclass transition showed upregulated metabolic processes. Newly diagnosed glioblastomas with mesenchymal transition displayed increased stem cell-like states and decreased immune components at diagnosis and exhibited elevated immune signatures and cytokine levels in serum. In contrast, tissue of recurrent glioblastomas with mesenchymal transition showed increased immune components but decreased stem cell-like states. Survival analyses revealed comparable outcomes for patients with and without subclass transitions. This study demonstrates a temporal heterogeneity of DNA methylation subclasses in 28.2% of glioblastomas, not impacting patient survival. Changes in cell state composition associated with subclass transition may be crucial for recurrent glioblastoma targeted therapies.
Keywords: DNA methylation; Deconvolution; Glioma; Outcome; Subgroup; Temporal.
© 2024. The Author(s).
Conflict of interest statement
M.L.S. is equity holder, scientific co-founder and advisory board member of Immunitas Therapeutics.
Figures


Similar articles
-
Peripheral blood-derived immune cell counts as prognostic indicators and their relationship with DNA methylation subclasses in glioblastoma patients.Brain Pathol. 2025 Jul;35(4):e13334. doi: 10.1111/bpa.13334. Epub 2025 Feb 3. Brain Pathol. 2025. PMID: 39901324 Free PMC article.
-
DNA methylation subclasses predict the benefit from gross total tumor resection in IDH-wildtype glioblastoma patients.Neuro Oncol. 2023 Feb 14;25(2):315-325. doi: 10.1093/neuonc/noac177. Neuro Oncol. 2023. PMID: 35868257 Free PMC article.
-
A Distinct DNA Methylation Shift in a Subset of Glioma CpG Island Methylator Phenotypes during Tumor Recurrence.Cell Rep. 2018 Apr 10;23(2):637-651. doi: 10.1016/j.celrep.2018.03.107. Cell Rep. 2018. PMID: 29642018 Free PMC article.
-
Congress of neurological surgeons systematic review and evidence-based guidelines update on the role of emerging developments in the management of newly diagnosed glioblastoma.J Neurooncol. 2020 Nov;150(2):269-359. doi: 10.1007/s11060-020-03607-4. Epub 2020 Nov 19. J Neurooncol. 2020. PMID: 33215345
-
Treatment considerations for MGMT-unmethylated glioblastoma.Curr Neurol Neurosci Rep. 2015 Jan;15(1):507. doi: 10.1007/s11910-014-0507-z. Curr Neurol Neurosci Rep. 2015. PMID: 25394859 Review.
Cited by
-
CTDSP2::GLI1 fusion in glioblastoma: A diagnostic challenge through tumor heterogeneity.J Neuropathol Exp Neurol. 2024 Dec 1;83(12):1076-1080. doi: 10.1093/jnen/nlae073. J Neuropathol Exp Neurol. 2024. PMID: 38981111 Free PMC article. No abstract available.
-
Valproic Acid and Celecoxib Enhance the Effect of Temozolomide on Glioblastoma Cells.CNS Neurol Disord Drug Targets. 2025;24(5):375-381. doi: 10.2174/0118715273330268241008220702. CNS Neurol Disord Drug Targets. 2025. PMID: 39428930
-
Peripheral blood-derived immune cell counts as prognostic indicators and their relationship with DNA methylation subclasses in glioblastoma patients.Brain Pathol. 2025 Jul;35(4):e13334. doi: 10.1111/bpa.13334. Epub 2025 Feb 3. Brain Pathol. 2025. PMID: 39901324 Free PMC article.
-
A novel model of glioblastoma recurrence to identify therapeutic vulnerabilities.EMBO Mol Med. 2025 Jun;17(6):1325-1354. doi: 10.1038/s44321-025-00237-z. Epub 2025 Apr 28. EMBO Mol Med. 2025. PMID: 40295888 Free PMC article.
-
Longitudinal multimodal profiling of IDH-wildtype glioblastoma reveals the molecular evolution and cellular phenotypes underlying prognostically different treatment responses.Neuro Oncol. 2025 Jan 12;27(1):89-105. doi: 10.1093/neuonc/noae214. Neuro Oncol. 2025. PMID: 39560080 Free PMC article.
References
-
- Al-kharboosh R, ReFaey K, Lara-Velazquez M, Grewal SS, Imitola J, Quiñones-Hinojosa A. Inflammatory mediators in glioma microenvironment play a dual role in gliomagenesis and mesenchymal stem cell homing: implication for cellular therapy. Mayo Clin Proc Innov Qual Outcomes. 2020;4:443–459. doi: 10.1016/j.mayocpiqo.2020.04.006. - DOI - PMC - PubMed
-
- Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, Kleinschmidt-DeMasters BK, Perry A, Reifenberger G, Stupp R, von Deimling A, Weller M. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol (Berl) 2018;136:805–810. doi: 10.1007/s00401-018-1913-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

