Expression and potential regulatory functions of Drosophila octopamine receptors in the female reproductive tract
- PMID: 38244217
- PMCID: PMC10917510
- DOI: 10.1093/g3journal/jkae012
Expression and potential regulatory functions of Drosophila octopamine receptors in the female reproductive tract
Abstract
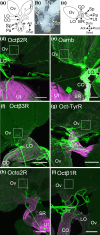
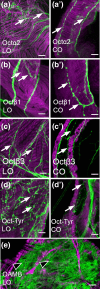
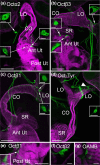
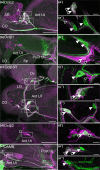
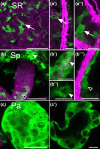
Aminergic signaling is known to play a critical role in regulating female reproductive processes in both mammals and insects. In Drosophila, the ortholog of noradrenaline, octopamine, is required for ovulation as well as several other female reproductive processes. Two octopamine receptors have already been shown to be expressed in the Drosophila reproductive tract and to be required for egg-laying: OAMB and Octβ2R. The Drosophila genome contains 4 additional octopamine receptors-Octα2R, Octβ1R, Octβ3R, and Oct-TyrR-but their cellular patterns of expression in the reproductive tract and potential contribution(s) to egg-laying are not known. In addition, the mechanisms by which OAMB and Octβ2R regulate reproduction are incompletely understood. Using a panel of MiMIC Gal4 lines, we show that Octα2R, Octβ1R, Octβ3R, and Oct-TyrR receptors are not detectable in either epithelium or muscle but are clearly expressed in neurons within the female fly reproductive tract. Optogenetic activation of neurons that express at least 3 types of octopamine receptors stimulates contractions in the lateral oviduct. We also find that octopamine stimulates calcium transients in the sperm storage organs and that its effects in spermathecal, secretory cells, can be blocked by knock-down of OAMB. These data extend our understanding of the pathways by which octopamine regulates egg-laying in Drosophila and raise the possibility that multiple octopamine receptor subtypes could play a role in this process.
Keywords: egg-laying; octopamine; octopamine receptor; oviposition; spermatheca.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures


Similar articles
-
Regulation of Drosophila oviduct muscle contractility by octopamine.iScience. 2022 Jul 2;25(8):104697. doi: 10.1016/j.isci.2022.104697. eCollection 2022 Aug 19. iScience. 2022. PMID: 35880044 Free PMC article.
-
The octopamine receptor Octβ2R regulates ovulation in Drosophila melanogaster.PLoS One. 2014 Aug 6;9(8):e104441. doi: 10.1371/journal.pone.0104441. eCollection 2014. PLoS One. 2014. PMID: 25099506 Free PMC article.
-
Drosophila cells that express octopamine receptors can either inhibit or promote oviposition.bioRxiv [Preprint]. 2023 May 3:2023.05.03.539296. doi: 10.1101/2023.05.03.539296. bioRxiv. 2023. PMID: 37205438 Free PMC article. Preprint.
-
Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera.Arch Insect Biochem Physiol. 2001 Sep;48(1):13-38. doi: 10.1002/arch.1055. Arch Insect Biochem Physiol. 2001. PMID: 11519073 Review.
-
Neurobiology of egg-laying behavior in Drosophila: neural control of the female reproductive system.J Neurogenet. 2024 Sep;38(3):47-61. doi: 10.1080/01677063.2024.2396352. Epub 2024 Sep 9. J Neurogenet. 2024. PMID: 39250036 Review.
Cited by
-
Heterogeneity in the projections and excitability of tyraminergic/octopaminergic neurons that innervate the Drosophila reproductive tract.Front Mol Neurosci. 2024 Aug 2;17:1374896. doi: 10.3389/fnmol.2024.1374896. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39156129 Free PMC article.
-
Two classes of amine/glutamate multi-transmitter neurons innervate Drosophila internal male reproductive organs.bioRxiv [Preprint]. 2025 Jul 28:2025.07.23.666348. doi: 10.1101/2025.07.23.666348. bioRxiv. 2025. PMID: 40766443 Free PMC article. Preprint.
-
Actomyosin contraction in the follicular epithelium provides the major mechanical force for follicle rupture during Drosophila ovulation.Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2407083121. doi: 10.1073/pnas.2407083121. Epub 2024 Sep 18. Proc Natl Acad Sci U S A. 2024. PMID: 39292751 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
