The LKB1-TSSK1B axis controls YAP phosphorylation to regulate the Hippo-YAP pathway
- PMID: 38245531
- PMCID: PMC10799855
- DOI: 10.1038/s41419-024-06465-4
The LKB1-TSSK1B axis controls YAP phosphorylation to regulate the Hippo-YAP pathway
Erratum in
-
Correction: The LKB1-TSSK1B axis controls YAP phosphorylation to regulate the Hippo-YAP pathway.Cell Death Dis. 2024 Nov 26;15(11):860. doi: 10.1038/s41419-024-07238-9. Cell Death Dis. 2024. PMID: 39592585 Free PMC article. No abstract available.
Abstract
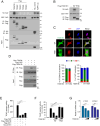
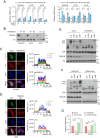
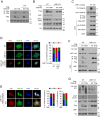
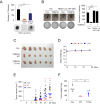
The Hippo pathway's main effector, Yes-associated protein (YAP), plays a crucial role in tumorigenesis as a transcriptional coactivator. YAP's phosphorylation by core upstream components of the Hippo pathway, such as mammalian Ste20 kinase 1/2 (MST1/2), mitogen-activated protein kinase kinase kinase kinases (MAP4Ks), and their substrate, large tumor suppressor 1/2 (LATS1/2), influences YAP's subcellular localization, stability, and transcriptional activity. However, recent research suggests the existence of alternative pathways that phosphorylate YAP, independent of these core upstream Hippo pathway components, raising questions about additional means to inactivate YAP. In this study, we present evidence demonstrating that TSSK1B, a calcium/calmodulin-dependent protein kinase (CAMK) superfamily member, is a negative regulator of YAP, suppressing cellular proliferation and oncogenic transformation. Mechanistically, TSSK1B inhibits YAP through two distinct pathways. Firstly, the LKB1-TSSK1B axis directly phosphorylates YAP at Ser94, inhibiting the YAP-TEAD complex's formation and suppressing its target genes' expression. Secondly, the TSSK1B-LATS1/2 axis inhibits YAP via phosphorylation at Ser127. Our findings reveal the involvement of TSSK1B-mediated molecular mechanisms in the Hippo-YAP pathway, emphasizing the importance of multilevel regulation in critical cellular decision-making processes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

