Real-world analysis of teclistamab in 123 RRMM patients from Germany
- PMID: 38245601
- PMCID: PMC10844072
- DOI: 10.1038/s41375-024-02154-5
Real-world analysis of teclistamab in 123 RRMM patients from Germany
Abstract
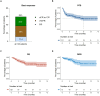
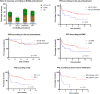
Teclistamab, a B-cell maturation antigen (BCMA) × CD3 directed bispecific antibody, has shown high response rates and durable remissions in the MAJESTEC-1 trial in patients with relapsed and refractory multiple myeloma (RRMM). We retrospectively assessed efficacy and tolerability in 123 patients treated at 18 different German centers to determine whether outcome is comparable in the real-world setting. Most patients had triple-class (93%) or penta-drug (60%) refractory disease, 37% of patients had received BCMA-directed pretreatment including idecabtagene vicleucel (ide-cel) CAR-T cell therapy (21/123, 17.1%). With a follow-up of 5.5 months, we observed an overall response rate (ORR) of 59.3% and a median progression-free survival (PFS) of 8.7 months. In subgroup analyses, we found significantly lower ORR and median PFS in patients with extramedullary disease (37%/2.1 months), and/or an ISS of 3 (37%/1.3 months), and ide-cel pretreated patients (33%/1.8 months). Nonetheless, the duration of response in ide-cel pretreated patients was comparable to that of anti-BCMA naive patients. Infections and grade ≥3 cytopenias were the most frequent adverse events. In summary, we found that teclistamab exhibited a comparable efficacy and safety profile in the real-world setting as in the pivotal trial.
© 2024. The Author(s).
Conflict of interest statement
BB has received honoraria from Janssen and GSK. MB has received speaker honoraria from AstraZeneca and Incyte and travel grants from Janssen. AC received consulting and/or lecture fees from Amgen, Janssen, Pfizer and Takeda and travel and congress participation grants from Janssen. HE has consulted for BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK and Novartis, he has received research funding by BMS/Celgene, Janssen, Amgen, GSK, Sanofi, honoraria from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, Novartis and travel support from BMS/Celgene, Janssen, Amgen, Takeda, Novartis, Sanofi. AN has consulted for Celgene, Roche, Takeda, Alexion, Janssen, BMS, Sanofi, Amgen and GSK. He received honoraria from Celgene, Roche, Takeda, Alexion, Janssen, BMS, Sanofi, Amgen, GSK and Jazz and research support from BMS, Janssen and Celgene. KMK received honoraria from from Abbvie, BMS, GSK, Janssen, Pfizer and Takeda. MM has received honoraria from Amgen, Takeda, BMS, Janssen, Stemline and Roche. MSR received honoraria and consulted for Janssen, BMS, Sanofi, Amgen, AbbVie and GSK. RT received honoraria and research funding from Janssen. RW consulted for Amgen, BMS/Celgene, Janssen, Novartis, Kite/Gilead, Pfizer, Sanofi and Takeda, he received research funding from Janssen and Sanofi, and travel support from Janssen, Kite/Gilead, Pfizer and BMS. LR consulted for Janssen, Amgen, GSK, Pfizer, BMS, Sanofi, and received honoraria from Janssen, GSK, Pfizer, BMS, Sanofi and received research funding from Skyline Dx and BMS. CR, FBa, FBr, JFa, JFr, DG, SG-M, MHä, MHo, JK, MK, TL, CM, RM, AM declare no potential competing interests.
Figures
References
-
- Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2023;41:1265–74. doi: 10.1200/JCO.22.00842. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

