Results of a randomised Phase II trial of olaparib, chemotherapy or olaparib and cediranib in patients with platinum-resistant ovarian cancer
- PMID: 38245661
- PMCID: PMC10951211
- DOI: 10.1038/s41416-023-02567-6
Results of a randomised Phase II trial of olaparib, chemotherapy or olaparib and cediranib in patients with platinum-resistant ovarian cancer
Abstract
Background: OCTOVA compared the efficacy of olaparib (O) versus weekly paclitaxel (wP) or olaparib + cediranib (O + C) in recurrent ovarian cancer (OC).
Aims: The main aim of the OCTOVA trial was to determine the progression-free survival (PFS) of olaparib (O) versus the oral combination of olaparib plus cediranib (O + C) and weekly paclitaxel (wP) in recurrent ovarian cancer (OC).
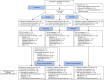
Methods: In total, 139 participants who had relapsed within 12 months of platinum therapy were randomised to O (300 mg twice daily), wP (80 mg/m2 d1,8,15, q28) or O + C (300 mg twice daily/20 mg daily, respectively). The primary endpoint was progression-free survival (PFS) of olaparib (O) versus olaparib plus cediranib (O + C) or weekly paclitaxel (wP). The sample size was calculated to observe a PFS hazard ratio (HR) 0.64 in favour of O + C compared to O (20% one-sided type I error, 80% power).
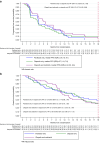
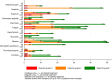
Results: The majority had platinum-resistant disease (90%), 22% prior PARPi, 34% prior anti-angiogenic therapy, 30% germline BRCA1/2 mutations. The PFS was increased for O + C vs O (O + C 5.4 mo (2.3, 9.6): O 3.7 mo (1.8, 7.6) HR = 0.73; 60% CI: 0.59, 0.89; P = 0.1) and no different between wP and O (wP 3.9 m (1.9, 9.1); O 3.7 mo (1.8, 7.6) HR = 0.89, 60% CI: 0.72, 1.09; P = 0.69). The main treatment-related adverse events included manageable diarrhoea (4% Grade 3) and hypertension (4% Grade 3) in the O + C arm.
Discussion: OCTOVA demonstrated the activity of O + C in women with recurrent disease, offering a potential non-chemotherapy option.
Trial registration: ISRCTN14784018, registered on 19th January 2018 http://www.isrctn.com/ISRCTN14784018 .
© 2024. The Author(s).
Conflict of interest statement
Competing interests for each author have been declared.
Figures

References
-
- Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84. doi: 10.1016/S1470-2045(17)30469-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

