Histone deacetylase inhibitors VPA and WT161 ameliorate the pathological features and cognitive impairments of the APP/PS1 Alzheimer's disease mouse model by regulating the expression of APP secretases
- PMID: 38245771
- PMCID: PMC10799458
- DOI: 10.1186/s13195-024-01384-0
Histone deacetylase inhibitors VPA and WT161 ameliorate the pathological features and cognitive impairments of the APP/PS1 Alzheimer's disease mouse model by regulating the expression of APP secretases
Abstract
Background: Alzheimer's disease (AD) is a degenerative neurological disorder. Recent studies have indicated that histone deacetylases (HDACs) are among the most prominent epigenetic therapy targets and that HDAC inhibitors have therapeutic effects on AD. Here, we identified sodium valproate (VPA), a pan-HDAC inhibitor, and WT161, a novel HDAC6 selective inhibitor, as potential therapeutic agents for AD. Underlying molecular mechanisms were investigated.
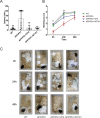
Methods: A cellular model, N2a-APPswe, was established via lentiviral infection, and the APPswe/PSEN1dE9 transgenic mouse model was employed in the study. LC-MS/MS was applied to quantify the concentration of WT161 in the mouse brain. Western blotting, immunohistochemical staining, thioflavin-S staining and ELISA were applied to detect protein expression in cells, tissues, or serum. RNA interference was utilized to knockdown the expression of specific genes in cells. The cognitive function of mice was assessed via the nest-building test, novel object recognition test and Morris water maze test.
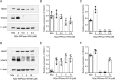
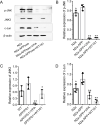
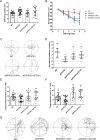
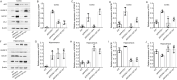
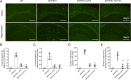
Results: Previous studies have focused mainly on the impact of HDAC inhibitors on histone deacetylase activity. Our study discovered that VPA and WT161 can downregulate the expression of multiple HDACs, such as HDAC1 and HDAC6, in both AD cell and mouse models. Moreover, they also affect the expression of APP and APP secretases (BACE1, PSEN1, ADAM10). RNA interference and subsequent vitamin C induction further confirmed that the expression of APP and APP secretases is indeed regulated by HDAC1 and HDAC6, with the JNK pathway being the intermediate link in this regulatory process. Through the above pathways, VPA and WT161 effectively reduced Aβ deposition in both AD cell and mouse models and significantly improved cognitive function in AD mice.
Conclusions: In general, we have discovered that the HDAC6-JNK-APP secretases cascade is an important pathway for VPA and WT161 to exert their therapeutic effects on AD. Investigations into the safety and efficacy of VPA and WT161 were also conducted, providing essential preclinical evidence for assessing these two epigenetic drugs for the treatment of AD.
Keywords: Alzheimer’s disease; Aβ deposition; Cognitive function; Histone deacetylase; VPA; WT161.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

