An efficient grafting method for phytoplasma transmission in Catharanthus roseus
- PMID: 38245775
- PMCID: PMC10799486
- DOI: 10.1186/s13007-024-01139-w
An efficient grafting method for phytoplasma transmission in Catharanthus roseus
Abstract
Background: Phytoplasmas are parasitic plant pathogens that reside intracellularly within the sieve tube cells. Phytoplasmas induce various symptoms, including floral virescence, phyllody, leaf yellowing, and witches'-broom. Currently, it is challenging to culture phytoplasma in vitro. In the laboratory, phytoplasmas are generally maintained in alternative host plants, such as Catharanthus roseus. Grafting is used to transmit phytoplasmas among the alternative hosts. During the experiment, scions from infected plants are grafted onto healthy plants using a side grafting method. However, the practice has certain limitations, including its inability to be applied to small plants and its irregular disease incidence.
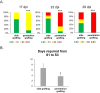
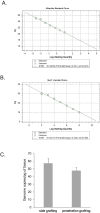
Results: Here, we demonstrate a new approach, penetration grafting, to overcome the limitations of side grafting. This grafting method allows phytoplasma to be efficiently and uniformly transmitted into the inoculated plants. No significant difference was observed in phytoplasma accumulation between both grafting techniques. However, penetration grafting allows rapid symptom development, saving waiting time and reducing space usage.
Conclusions: This study provides a reliable and stable method for experiments that require grafting transmission.
Keywords: Catharanthus roseus; Grafting; Phytoplasma symptom; Phytoplasma transmission.
© 2024. The Author(s).
Figures


Similar articles
-
Phytoplasma-induced floral abnormalities in Catharanthus roseus are associated with phytoplasma accumulation and transcript repression of floral organ identity genes.Mol Plant Microbe Interact. 2011 Dec;24(12):1502-12. doi: 10.1094/MPMI-06-11-0176. Mol Plant Microbe Interact. 2011. PMID: 21864044
-
First Report of Natural Infection by "Candidatus Phytoplasma brasiliense" in Catharanthus roseus.Plant Dis. 2001 Nov;85(11):1209. doi: 10.1094/PDIS.2001.85.11.1209C. Plant Dis. 2001. PMID: 30823182
-
Clover Proliferation Group (16SrVI) Subgroup A (16SrVI-A) Phytoplasma is a Probable Causal Agent of Dry Bean Phyllody Disease in Washington.Plant Dis. 2004 Apr;88(4):429. doi: 10.1094/PDIS.2004.88.4.429A. Plant Dis. 2004. PMID: 30812641
-
The genome biology of phytoplasma: modulators of plants and insects.Curr Opin Microbiol. 2012 Jun;15(3):247-54. doi: 10.1016/j.mib.2012.04.002. Epub 2012 Apr 28. Curr Opin Microbiol. 2012. PMID: 22542641 Review.
-
Global Status of Phytoplasma Diseases in Vegetable Crops.Front Microbiol. 2019 Jun 27;10:1349. doi: 10.3389/fmicb.2019.01349. eCollection 2019. Front Microbiol. 2019. PMID: 31316474 Free PMC article. Review.
Cited by
-
Status and Distribution of Diseases Caused by Phytoplasmas in Africa.Microorganisms. 2025 May 27;13(6):1229. doi: 10.3390/microorganisms13061229. Microorganisms. 2025. PMID: 40572118 Free PMC article. Review.
References
-
- Chang C-M, Jan F-J, Su C-C. Identification and characterization of phyllody phytoplasma associated with Vigna sesquipedalis in Taiwan. Taiwan Pesticide Science, 2018(4): p. 53–67.
-
- Bertaccini A, et al. Phytoplasmas and phytoplasma diseases: a severe threat to agriculture. Am J Plant Sci. 2014;05(12):26. doi: 10.4236/ajps.2014.512191. - DOI
-
- Liu H-L, Chen C-C, Lin C-P. Detection and identification of the phytoplasma associated with pear decline in Taiwan. Eur J Plant Pathol. 2007;117(3):281–91. doi: 10.1007/s10658-006-9094-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

