Chalcone-derivative L6H21 attenuates the OVA-induced asthma by targeting MD2
- PMID: 38245791
- PMCID: PMC10799361
- DOI: 10.1186/s40001-023-01630-5
Chalcone-derivative L6H21 attenuates the OVA-induced asthma by targeting MD2
Abstract
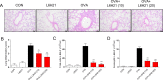
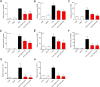
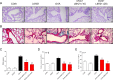
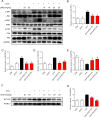
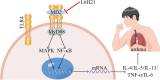
Asthma represents a significant global challenge that affects individuals across all age groups and imposes substantial social and economic burden. Due to heterogeneity of the disease, not all patients obtain benefit with current treatments. The objective of this study was to explore the impact of MD2 on the progression of asthma using L6H21, a novel MD2 inhibitor, to identify potential targets and drug candidates for asthma treatment. To establish an asthma-related murine model and evaluate the effects of L6H21, ovalbumin (OVA) was used to sensitize and challenge mice. Pathological changes were examined with various staining techniques, such as H&E staining, glycogen staining, and Masson staining. Inflammatory cell infiltration and excessive cytokine secretion were evaluated by analyzing BALF cell count, RT-PCR, and ELISA. The TLR4/MD2 complex formation, as well as the activation of the MAPK and NF-кB pathways, was examined using western blot and co-IP. Treatment with L6H21 demonstrated alleviation of increased airway resistance, lung tissue injury, inflammatory cell infiltration and excessive cytokine secretion triggered by OVA. In addition, it also ameliorated mucus production and collagen deposition. In the L6H21 treatment group, inhibition of MAPK and NF-кB activation was observed, along with the disruption of TLR4/MD2 complex formation, in contrast to the model group. Thus, L6H21 effectively reduced the formation of the MD2 and TLR4 complex induced by OVA in a dose-dependent manner. This reduction resulted in the attenuation of MAPKs/NF-κB activation, enhanced suppression of inflammatory factor secretion, reduced excessive recruitment of inflammatory cells, and ultimately mitigated airway damage. MD2 emerges as a crucial target for asthma treatment, and L6H21, as an MD2 inhibitor, shows promise as a potential drug candidate for the treatment of asthma.
Keywords: Asthma; Chalcone derivative; MD2 inhibitor; MD2 recombination; TLR4.
© 2024. The Author(s).
Conflict of interest statement
The author reports no competing interests in this work.
Figures


Similar articles
-
Myeloid Differentiation Protein 2 Mediates Angiotensin II-Induced Liver Inflammation and Fibrosis in Mice.Molecules. 2019 Dec 19;25(1):25. doi: 10.3390/molecules25010025. Molecules. 2019. PMID: 31861702 Free PMC article.
-
Glucagon Like Peptide-1 (GLP-1) Modulates OVA-Induced Airway Inflammation and Mucus Secretion Involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-κB (NF-κB) Signaling Pathway in Mice.Int J Mol Sci. 2015 Aug 26;16(9):20195-211. doi: 10.3390/ijms160920195. Int J Mol Sci. 2015. PMID: 26343632 Free PMC article.
-
Dexmedetomidine reduces IL-4 and IgE expression through downregulation of theTLR4/NF-κB signaling pathway to alleviate airway hyperresponsiveness in OVA mice.Pulm Pharmacol Ther. 2022 Aug;75:102147. doi: 10.1016/j.pupt.2022.102147. Epub 2022 Jul 19. Pulm Pharmacol Ther. 2022. PMID: 35863724
-
[Lung injury in ovalbumin-challenged asthma mice induced by high-dose PM2.5 and its mechanism].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017 Oct;33(10):1297-1302. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017. PMID: 29169411 Chinese.
-
Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets.Pharmacol Ther. 2018 Jan;181:169-182. doi: 10.1016/j.pharmthera.2017.08.011. Epub 2017 Aug 23. Pharmacol Ther. 2018. PMID: 28842273 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

