Achievement of Clinical, Endoscopic, and Histological Outcomes in Patients with Ulcerative Colitis Treated with Etrasimod, and Association with Faecal Calprotectin and C-reactive Protein: Results From the Phase 2 OASIS Trial
- PMID: 38245818
- PMCID: PMC11147797
- DOI: 10.1093/ecco-jcc/jjae007
Achievement of Clinical, Endoscopic, and Histological Outcomes in Patients with Ulcerative Colitis Treated with Etrasimod, and Association with Faecal Calprotectin and C-reactive Protein: Results From the Phase 2 OASIS Trial
Abstract
Background and aims: Etrasimod is an oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis [UC]. This post-hoc analysis of the phase 2 OASIS trial [NCT02447302] evaluated its efficacy for endoscopic improvement-histologic remission [EIHR] and assessed correlation between faecal calprotectin [FCP] and C-reactive protein [CRP] levels with efficacy outcomes.
Methods: In total, 156 adults with moderately to severely active UC received once-daily etrasimod (1 mg [n = 52]; 2 mg [n = 50]) or placebo [n = 54] for 12 weeks. Clinical, endoscopic, and histologic variables were evaluated at baseline and Week 12. EIHR was defined as achievement of endoscopic improvement [endoscopic subscore ≤ 1, without friability] and histologic remission [Geboes score < 2.0]. Outcomes included the relationships between FCP and CRP concentration and clinical, endoscopic, and histologic variables.
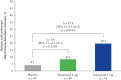
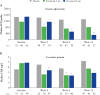
Results: Achievement of EIHR was significantly higher in patients who received etrasimod 2 mg versus placebo [19.5% vs 4.1%; Mantel-Haenszel estimated difference, 15.4%; p = 0.010]. In the etrasimod 2 mg group, median FCP and CRP levels at Week 12 were significantly lower in patients who achieved clinical remission, endoscopic improvement, histologic remission, and EIHR versus patients who did not [all p < 0.05]. An FCP concentration cutoff of 250 µg/g achieved optimum sensitivity and specificity for efficacy, including EIHR [0.857 and 0.786, respectively; κ coefficient, 0.3584]. Higher proportions of patients with FCP ≤ 250 µg/g achieved efficacy outcomes at Week 12 versus patients with FCP > 250 µg/g.
Conclusions: Etrasimod was effective for inducing EIHR in patients with UC. FCP and CRP may be useful, noninvasive biomarkers to monitor treatment response.
Clinicaltrials.gov number: NCT02447302.
Keywords: Etrasimod; S1P receptor modulator; endoscopic improvement–histologic remission; mucosal healing; ulcerative colitis.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
A.J.Y. has received consulting fees from Takeda, Prometheus Labs, Arena Pharmaceuticals, Inc., and Bristol Myers Squibb; and speakers’ bureau fees from Bristol Myers Squibb. M.V.C. has been a speaker for AbbVie, Janssen, Medtronic, Pfizer Inc., and Takeda; and has served as a consultant for AbbVie, Arena Pharmaceuticals, Inc., Bristol Myers Squibb, Medtronic, Pfizer Inc., Lilly, Prometheus, Fresenius Kabi, and Takeda; and grant support from Pfizer, Janssen, Novartis, and Bristol Myers Squibb. J.P. has received consulting fees from AbbVie, Arena Pharmaceuticals, Inc., Athos, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Genentech, GlaxoSmithKline, Janssen, Mirum, Morphic, Nestlé, Origo, Pandion, Progenity, Pfizer, Revolo, Robarts, Roche, Takeda, Theravance Biopharma, and Wassermann; and speaker fees from AbbVie, Biogen, Ferring, Janssen, Pfizer, and Takeda; and research funding from AbbVie and Pfizer. V.J. has received consulting/advisory board fees from AbbVie, Alimentiv Inc., Arena Pharmaceuticals, Inc., Asahi Kasei Pharma, Asieris, AstraZeneca, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, GlaxoSmithKline, Genentech, Gilead Sciences, Janssen, Merck, Metacrine, Mylan, Pandion, Pendopharm, Pfizer, Protagonist, Prometheus, Reistone Biopharma, Roche, Sandoz, Second Genome, Sorriso Pharmaceuticals, Takeda, Teva, TopiVert, Ventyx, and Vividion; and speaker’s fees from AbbVie, Ferring, Bristol Myers Squibb, Galapagos, Janssen, Pfizer, Shire Pharmaceuticals, Takeda, and Fresenius Kabi. J.Z. is an employee of Arena Pharmaceuticals, Inc., a wholly owned subsidiary of Pfizer Inc, New York, NY. C.J.R. is an employee of Arena Pharmaceuticals, Inc., a wholly owned subsidiary of Pfizer Inc, New York, NY. W.J.S. has received research grants from AbbVie, Abivax, Arena Pharmaceuticals,Inc., Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Lilly, Pfizer, Prometheus Laboratories, Seres Therapeutics, Shire Pharmaceuticals, Takeda, and Theravance Biopharma; consulting fees from AbbVie, Abivax, Alfasigma, Alimentiv [previously Robarts Clinical Trials, owned by Alimentiv Health Trust], Allakos, Amgen, Arena Pharmaceuticals, Inc., AstraZeneca, Atlantic Pharmaceuticals, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, ClostraBio, Forbion, Galapagos, Genentech [Roche], GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Iota Biosciences, Janssen, Lilly, Morphic Therapeutics, Novartis, Oppilan Pharma [now Ventyx Biosciences], Pfizer, Pharm Olam, Polpharma, Progenity, Prometheus Biosciences, Protagonist Therapeutics, PTM Therapeutics, Seres Therapeutics, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Vedanta Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivreon Gastrosciences, Xencor, and Zealand Pharmaceuticals; owns stock or stock options in Allakos, BeiGene, Gossamer Bio, Oppilan Pharma [now Ventyx Biosciences], Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, and Vivreon Gastrosciences; and is chief medical officer at Ventyx Biosciences. S.V. has received grants from AbbVie, Johnson & Johnson, Pfizer, Galapagos, and Takeda; and has received consulting and/or speaker fees from AbbVie, AbolerIS Pharma, Agomab, Alimentiv, Arena Pharmaceuticals, Inc., AstraZeneca, Avaxia, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, CVasThera, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead Sciences, GlaxoSmithKline, Hospira, IMIDomics, Janssen, Johnson & Johnson, Lilly, Materia Prima, MiroBio, Morphic, MRM Health, Mundipharma, MSD, Pfizer, ProDigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire Pharmaceuticals, Surrozen, Takeda, Theravance Biopharma, Tillotts Pharma AG, and Zealand Pharma. L.P.B. has served as a consultant for AbbVie, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Inc., Biogen, Bristol Myers Squibb, Celltrion, CONNECT Biopharm, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead Sciences, Gossamer Bio, GlaxoSmithKline, HAC-Pharma, IAG Image Analysis, Index Pharmaceuticals, Inotrem, Janssen, Lilly, Medac, Mopac, Morphic, MSD, Norgine, Novartis, OM Pharma, ONO Pharma, OSE Immunotherapeutics, Pandion Therapeutics, Pfizer, Prometheus, Protagonist, Roche, Sandoz, Takeda, Theravance Biopharma, Thermo Fisher, Tigenix, Tillots, Viatris, Vifor, Ysopia, and Abivax; received grants from Takeda, Fresenius Kabi, and Celltrion; and has received lecture/speaker fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillotts, Celltrion, Takeda, Pfizer, Sandoz, Biogen, MSD, Amgen, Vifor, Arena Pharmaceuticals, Inc., Lilly, Gilead Sciences, Viatris, and Medac.
Figures


References
-
- Moriichi K, Fujiya M, Okumura T.. The endoscopic diagnosis of mucosal healing and deep remission in inflammatory bowel disease. Dig Endosc 2021;33:1008–23. - PubMed
-
- Mazzuoli S, Guglielmi FW, Antonelli E, Salemme M, Bassotti G, Villanacci V.. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis 2013;45:969–77. - PubMed
-
- Danese S, Roda G, Peyrin-Biroulet L.. Evolving therapeutic goals in ulcerative colitis: towards disease clearance. Nat Rev Gastroenterol Hepatol 2020;17:1–2. - PubMed
-
- Sandborn WJ, Feagan BG, D’Haens G, et al.; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2021;385:1280–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

