Single-Response Duplexing of Electrochemical Label-Free Biosensor from the Same Tag
- PMID: 38245830
- PMCID: PMC11468374
- DOI: 10.1002/adhm.202303509
Single-Response Duplexing of Electrochemical Label-Free Biosensor from the Same Tag
Abstract
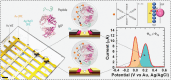
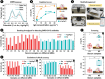
Multiplexing is a valuable strategy to boost throughput and improve clinical accuracy. Exploiting the vertical, meshed design of reproducible and low-cost ultra-dense electrochemical chips, the unprecedented single-response multiplexing of typical label-free biosensors is reported. Using a cheap, handheld one-channel workstation and a single redox probe, that is, ferro/ferricyanide, the recognition events taking place on two spatially resolved locations of the same working electrode can be tracked along a single voltammetry scan by collecting the electrochemical signatures of the probe in relation to different quasi-reference electrodes, Au (0 V) and Ag/AgCl ink (+0.2 V). This spatial isolation prevents crosstalk between the redox tags and interferences over functionalization and binding steps, representing an advantage over the existing non-spatially resolved single-response multiplex strategies. As proof of concept, peptide-tethered immunosensors are demonstrated to provide the duplex detection of COVID-19 antibodies, thereby doubling the throughput while achieving 100% accuracy in serum samples. The approach is envisioned to enable broad applications in high-throughput and multi-analyte platforms, as it can be tailored to other biosensing devices and formats.
Keywords: accuracy; multiplexed detection; serology; single‐channel potentiostat; square wave voltammetry; steric hindrance; throughput.
© 2024 The Authors. Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
R.S.L, J.N.Y.C., G.J.C.P., A.L.G., M.H.O.P., and F.M.S. are listed as inventors on a patent filing application describing the microfabrication presented here. The remaining authors declare no conflict of interest.
Figures



Similar articles
-
Ultradense Array of On-Chip Sensors for High-Throughput Electrochemical Analyses.ACS Sens. 2024 Aug 23;9(8):4089-4097. doi: 10.1021/acssensors.4c01026. Epub 2024 Jul 12. ACS Sens. 2024. PMID: 38997236
-
Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen.Mikrochim Acta. 2021 May 26;188(6):199. doi: 10.1007/s00604-021-04867-1. Mikrochim Acta. 2021. PMID: 34041585 Free PMC article.
-
An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes.Mikrochim Acta. 2019 Mar 7;186(4):224. doi: 10.1007/s00604-019-3345-5. Mikrochim Acta. 2019. PMID: 30847572 Free PMC article.
-
The potential application of electrochemical biosensors in the COVID-19 pandemic: A perspective on the rapid diagnostics of SARS-CoV-2.Biosens Bioelectron. 2021 Mar 15;176:112905. doi: 10.1016/j.bios.2020.112905. Epub 2020 Dec 17. Biosens Bioelectron. 2021. PMID: 33358285 Free PMC article. Review.
-
Beyond traditional biosensors: Recent advances in gold nanoparticles modified electrodes for biosensing applications.Talanta. 2024 Feb 1;268(Pt 1):125280. doi: 10.1016/j.talanta.2023.125280. Epub 2023 Oct 12. Talanta. 2024. PMID: 37862755 Review.
Cited by
-
Scalable electrochemical system for rapid on-site detection of food allergens.Biosens Bioelectron. 2025 Apr 1;273:117142. doi: 10.1016/j.bios.2025.117142. Epub 2025 Jan 13. Biosens Bioelectron. 2025. PMID: 39832405
-
Advanced Neuromorphic Applications Enabled by Synaptic Ion-Gating Vertical Transistors.Adv Sci (Weinh). 2024 Jul;11(27):e2305611. doi: 10.1002/advs.202305611. Epub 2024 May 17. Adv Sci (Weinh). 2024. PMID: 38757653 Free PMC article. Review.
-
Colorectal Cancer Label-Free Impedimetric Immunosensor for Blood-Based Biomarker CCSP-2.ACS Meas Sci Au. 2025 Feb 5;5(1):87-95. doi: 10.1021/acsmeasuresciau.4c00073. eCollection 2025 Feb 19. ACS Meas Sci Au. 2025. PMID: 39991036 Free PMC article.
-
Touch-Enabled Reversible Microfluidic Ultradense Chips for Convenient, High-Throughput Electrochemical Assays.ACS Appl Mater Interfaces. 2025 Aug 13;17(32):45847-45858. doi: 10.1021/acsami.5c08760. Epub 2025 Jul 21. ACS Appl Mater Interfaces. 2025. PMID: 40686371 Free PMC article.
References
-
- Welch E. C., Powell J. M., Clevinger T. B., Fairman A. E., Shukla A., Adv. Funct. Mater. 2021, 31, 2104126.
-
- Kabay G., DeCastro J., Altay A., Smith K., Lu H. W., Capossela A. M. D., Moarefian M., Aran K., Dincer C., Adv. Mater. 2022, 34, 2201085. - PubMed
-
- Zhang Z., Ma P., Ahmed R., Wang J., Akin D., Soto F., Liu B. F., Li P., Demirci U., Adv. Mater. 2022, 34, 2103646. - PubMed
-
- Clifford A., Das J., Yousefi H., Mahmud A., Chen J. B., Kelley S. O., J. Am. Chem. Soc. 2021, 143, 5281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022/04397-1/São Paulo Research Foundation
- 2023/02080-3/São Paulo Research Foundation
- 88887.663312/2022-00/Brazilian Coordination for Improvement of Higher Education Personnel
- 407951/2021-0/Brazilian National Council for Scientific and Technological Development
- 304389/2019-6/Brazilian National Council for Scientific and Technological Development
LinkOut - more resources
Full Text Sources
Medical
Research Materials

