Development and Validation of the Phoenix Criteria for Pediatric Sepsis and Septic Shock
- PMID: 38245897
- PMCID: PMC10900964
- DOI: 10.1001/jama.2024.0196
Development and Validation of the Phoenix Criteria for Pediatric Sepsis and Septic Shock
Abstract
Importance: The Society of Critical Care Medicine Pediatric Sepsis Definition Task Force sought to develop and validate new clinical criteria for pediatric sepsis and septic shock using measures of organ dysfunction through a data-driven approach.
Objective: To derive and validate novel criteria for pediatric sepsis and septic shock across differently resourced settings.
Design, setting, and participants: Multicenter, international, retrospective cohort study in 10 health systems in the US, Colombia, Bangladesh, China, and Kenya, 3 of which were used as external validation sites. Data were collected from emergency and inpatient encounters for children (aged <18 years) from 2010 to 2019: 3 049 699 in the development (including derivation and internal validation) set and 581 317 in the external validation set.
Exposure: Stacked regression models to predict mortality in children with suspected infection were derived and validated using the best-performing organ dysfunction subscores from 8 existing scores. The final model was then translated into an integer-based score used to establish binary criteria for sepsis and septic shock.
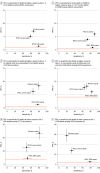
Main outcomes and measures: The primary outcome for all analyses was in-hospital mortality. Model- and integer-based score performance measures included the area under the precision recall curve (AUPRC; primary) and area under the receiver operating characteristic curve (AUROC; secondary). For binary criteria, primary performance measures were positive predictive value and sensitivity.
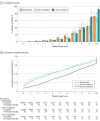
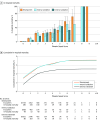
Results: Among the 172 984 children with suspected infection in the first 24 hours (development set; 1.2% mortality), a 4-organ-system model performed best. The integer version of that model, the Phoenix Sepsis Score, had AUPRCs of 0.23 to 0.38 (95% CI range, 0.20-0.39) and AUROCs of 0.71 to 0.92 (95% CI range, 0.70-0.92) to predict mortality in the validation sets. Using a Phoenix Sepsis Score of 2 points or higher in children with suspected infection as criteria for sepsis and sepsis plus 1 or more cardiovascular point as criteria for septic shock resulted in a higher positive predictive value and higher or similar sensitivity compared with the 2005 International Pediatric Sepsis Consensus Conference (IPSCC) criteria across differently resourced settings.
Conclusions and relevance: The novel Phoenix sepsis criteria, which were derived and validated using data from higher- and lower-resource settings, had improved performance for the diagnosis of pediatric sepsis and septic shock compared with the existing IPSCC criteria.
Conflict of interest statement
Figures

Comment in
-
Context and Implications of the New Pediatric Sepsis Criteria.JAMA. 2024 Feb 27;331(8):646-649. doi: 10.1001/jama.2023.27979. JAMA. 2024. PMID: 38245890 No abstract available.
-
Transitioning From SIRS to Phoenix With the Updated Pediatric Sepsis Criteria-The Difficult Task of Simplifying the Complex.JAMA. 2024 Feb 27;331(8):650-651. doi: 10.1001/jama.2023.27988. JAMA. 2024. PMID: 38245901 No abstract available.
-
Phoenix Criteria for Pediatric Sepsis and Septic Shock.JAMA. 2024 Jun 18;331(23):2049-2050. doi: 10.1001/jama.2024.8199. JAMA. 2024. PMID: 38780925 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

