Mesonephric and mesonephric-like adenocarcinomas of gynecologic origin: A single-center experience with molecular characterization, treatment, and oncologic outcomes
- PMID: 38246044
- PMCID: PMC10960687
- DOI: 10.1016/j.ygyno.2024.01.015
Mesonephric and mesonephric-like adenocarcinomas of gynecologic origin: A single-center experience with molecular characterization, treatment, and oncologic outcomes
Abstract
Objectives: Mesonephric (MA) and mesonephric-like (MLA) adenocarcinomas are rare cancers, and data on clinical behavior and response to therapy are limited. We sought to report molecular features, treatment, and outcomes of MA/MLA from a single institution.
Methods: Patients with MA (cervix) or MLA (uterus, ovary, other) treated at Memorial Sloan Kettering Cancer Center (MSK) from 1/2008-12/2021 underwent pathologic re-review. For patients with initial treatment at MSK, progression-free survival (PFS1) was calculated as time from initial surgery to progression or death; second PFS (PFS2) was calculated as time from start of treatment for recurrence to subsequent progression or death. Overall survival (OS) was calculated for all patients. Images were retrospectively reviewed to determine treatment response. Somatic genetic alterations were assessed by clinical tumor-normal sequencing (MSK-Integrated Mutation Profiling of Actionable Cancer Targets [MSK-IMPACT]).
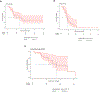
Results: Of 81 patients with confirmed gynecologic MA/MLA, 36 received initial treatment at MSK. Sites of origin included cervix (n = 9, 11%), uterus (n = 42, 52%), ovary (n = 28, 35%), and other (n = 2, 2%). Of the 36 patients who received initial treatment at MSK, 20 (56%) recurred; median PFS1 was 33 months (95% CI: 17-not evaluable), median PFS2 was 8.3 months (95% CI: 6.9-14), and median OS was 87 months (95% CI: 58.2-not evaluable). Twenty-six of the 36 patients underwent MSK-IMPACT testing, and 25 (96%) harbored MAPK pathway alterations.
Conclusion: Most patients diagnosed with early-stage disease ultimately recurred. Somatic MAPK signaling pathway mutations appear to be highly prevalent in MA/MLA, and therapeutics that target this pathway are worthy of further study.
Keywords: KRAS; MAPK; Mesonephric adenocarcinoma; Mesonephric-like adenocarcinoma.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest B. Weigelt reports research funding by Repare Therapeutics, outside the current work. N. R. Abu-Rustum reports grant funding from GRAIL paid to the institution. A. Iasonos reports consulting fees from Mylan. C. Aghajanian reports clinical trial funding paid to the institution from AstraZeneca; consulting fees (advisory board) from Eisai/Merck, Roche/Genentech, Abbvie, AstraZeneca/Merck, and Repare Therapeutics; advisory board participation (no fee) for Blueprint Medicine; and leadership/fiduciary roles for the GOG Foundation Board of Directors (travel cost reimbursement) and NRG Oncology Board of Directors (unpaid). D. Chi reports personal fees from Apyx Medical, Verthermia Inc., Biom ‘Up, and AstraZeneca, as well as recent or current stock/options ownership of Apyx Medical, Verthemia, Intuitive Surgical, Inc., TransEnterix, Inc., Doximity, Moderna, and BioNTech SE. R.N. Grisham reports honoraria from GSK, AstraZeneca, Natera, Springworks, Corcept, MJH, and PER. The other authors do not have potential conflicts of interest to declare.
Figures


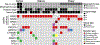
References
-
- Mirkovic J, et al., Targeted Genomic Profiling Reveals Recurrent KRAS Mutations in Mesonephric-like Adenocarcinomas of the Female Genital Tract. Am J Surg Pathol, 2018. 42(2): p. 227–233. - PubMed
-
- Clement PB, et al., Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a malignant spindle cell component. Am J Surg Pathol, 1995. 19(10): p. 1158–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

