Dietary Iron Is Necessary to Support Proliferative Regeneration after Intestinal Injury
- PMID: 38246358
- PMCID: PMC11181351
- DOI: 10.1016/j.tjnut.2024.01.013
Dietary Iron Is Necessary to Support Proliferative Regeneration after Intestinal Injury
Abstract
Background: Tissue repair and regeneration in the gastrointestinal system are crucial for maintaining homeostasis, with the process relying on intricate cellular interactions and affected by micro- and macro-nutrients. Iron, essential for various biological functions, plays a dual role in tissue healing by potentially causing oxidative damage and participating in anti-inflammatory mechanisms, underscoring its complex relationship with inflammation and tissue repair.
Objective: The study aimed to elucidate the role of low dietary iron in gastrointestinal tissue repair.
Methods: We utilized quantitative iron measurements to assess iron levels in inflamed regions of patients with ulcerative colitis and Crohn's disease. In addition, 3 mouse models of gastrointestinal injury/repair (dextran sulfate sodium-induced colitis, radiation injury, and wound biopsy) were used to assess the effects of low dietary iron on tissue repair.
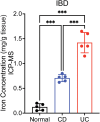
Results: We found that levels of iron in inflamed regions of both patients with ulcerative colitis and Crohn's disease are elevated. Similarly, during gastrointestinal repair, iron levels were found to be heightened, specifically in intestinal epithelial cells across the 3 injury/repair models. Mice on a low-iron diet showed compromised tissue repair with reduced proliferation. In standard diet, epithelial cells and the stem cell compartment maintain adequate iron stores. However, during a period of iron deficiency, epithelial cells exhaust their iron reserves, whereas the stem cell compartments maintain their iron pools. During injury, when the stem compartment is disrupted, low iron levels impair proliferation and compromise repair mechanisms.
Conclusions: Low dietary iron impairs intestinal repair through compromising the ability of epithelial cells to aid in intestinal proliferation.
Keywords: intestinal injury; intestinal proliferation; iron; tissue repair; wound healing.
Copyright © 2024 American Society for Nutrition. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Palmieri B., Vadalà M., Laurino C. Nutrition in wound healing: investigation of the molecular mechanisms, a narrative review. J. Wound Care. 2019;28:683–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

