Identification of AgRP cells in the murine hindbrain that drive feeding
- PMID: 38246589
- PMCID: PMC10844855
- DOI: 10.1016/j.molmet.2024.101886
Identification of AgRP cells in the murine hindbrain that drive feeding
Abstract
Objective: The central melanocortin system is essential for the regulation of food intake and body weight. Agouti-related protein (AgRP) is the sole orexigenic component of the central melanocortin system and is conserved across mammalian species. AgRP is currently known to be expressed exclusively in the mediobasal hypothalamus, and hypothalamic AgRP-expressing neurons are essential for feeding. Here we characterized a previously unknown population of AgRP cells in the mouse hindbrain.
Methods: Expression of AgRP in the hindbrain was investigated using gene expression analysis, single-cell RNA sequencing, immunofluorescent analysis and multiple transgenic mice with reporter expressions. Activation of AgRP neurons was achieved by Designer Receptors Exclusively Activated by Designer Drugs (DREADD) and by transcranial focal photo-stimulation using a step-function opsin with ultra-high light sensitivity (SOUL).
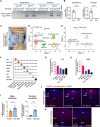
Results: AgRP expressing cells were present in the area postrema (AP) and the adjacent subpostrema area (SubP) and commissural nucleus of the solitary tract (cNTS) of the mouse hindbrain (termed AgRPHind herein). AgRPHind cells consisted of locally projecting neurons as well as tanycyte-like cells. Food deprivation stimulated hindbrain Agrp expression as well as neuronal activity of subsets of AgRPHind cells. In adult mice that lacked hypothalamic AgRP neurons, chemogenetic activation of AgRP neurons resulted in hyperphagia and weight gain. In addition, transcranial focal photo-stimulation of hindbrain AgRP cells increased food intake in adult mice with or without hypothalamic AgRP neurons.
Conclusions: Our study indicates that the central melanocortin system in the hindbrain possesses an orexigenic component, and that AgRPHind neurons stimulate feeding independently of hypothalamic AgRP neurons.
Keywords: AgRP; Feeding; Hindbrain.
Copyright © 2024 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Ollmann M.M., Wilson B.D., Yang Y.K., Kerns J.A., Chen Y., Gantz I., et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. - PubMed
-
- Rossi M., Kim M.S., Morgan D.G., Small C.J., Edwards C.M., Sunter D., et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–4431. - PubMed

