Rare, late onset of immune checkpoint inhibitor-induced type 1 diabetes mellitus in a patient with small-cell lung cancer treated with serplulimab: a case report and review of the literature
- PMID: 38247005
- PMCID: PMC10801956
- DOI: 10.1186/s13256-023-04248-7
Rare, late onset of immune checkpoint inhibitor-induced type 1 diabetes mellitus in a patient with small-cell lung cancer treated with serplulimab: a case report and review of the literature
Abstract
Background: As a newly approved immune checkpoint inhibitor in China, serplulimab has been widely used in the immunotherapy of tumors. However, the immune-related adverse events of immune checkpoint inhibitors should not be ignored. Although immune checkpoint inhibitor-induced type 1 diabetes mellitus is a rare complication, it may cause diabetic ketoacidosis and endanger the lives of patients.
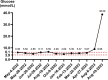
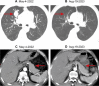
Case presentation: This case report describes a 55-year-old male of Han nationality from China diagnosed with small-cell lung cancer with multiple metastases who experienced an adverse event of type 1 diabetes mellitus 68 weeks after receiving serplulimab therapy. The patient presented with typical symptoms of diabetic ketoacidosis, including severe thirst, nausea, vomiting, deep respirations, and stupor. Despite the absence of diabetes-related autoantibodies, the patient had extremely low levels of insulin and C-peptide release. Other potential causes of diabetes were ruled out, confirming the condition as serplulimab-induced immune checkpoint inhibitor-induced type 1 diabetes mellitus. After aggressive treatment to correct diabetic ketoacidosis, the patient's blood glucose levels stabilized and symptoms of diabetes improved significantly, although long-term insulin maintenance therapy was necessary.
Conclusion: This case highlights a rare, late-onset adverse event of immune checkpoint inhibitor-induced type 1 diabetes mellitus that may be overlooked during treatment with serplulimab. The monitoring of blood glucose levels and early signs and symptoms of diabetes cannot be relaxed at the late stage of treatment, even if patients do not have elevated blood glucose levels before and during the middle stage of treatment.
Keywords: Case report; Immune-checkpoint inhibitor–induced type 1 diabetes mellitus; Late-onset adverse events; Serplulimab; Small-cell lung cancer; Treatment management.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer.J Immunother Cancer. 2017 May 16;5:40. doi: 10.1186/s40425-017-0245-2. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515940 Free PMC article.
-
Immune checkpoint inhibitor-associated diabetic ketoacidosis and insulin-dependent diabetes: a case report.J Med Case Rep. 2024 Dec 27;18(1):611. doi: 10.1186/s13256-024-04852-1. J Med Case Rep. 2024. PMID: 39726058 Free PMC article.
-
Immune Checkpoint Inhibitor-Induced Diabetic Ketoacidosis: A Report of Four Cases and Literature Review.Front Endocrinol (Lausanne). 2020 Jan 28;11:14. doi: 10.3389/fendo.2020.00014. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32047478 Free PMC article. Review.
-
New-onset type 1 diabetes mellitus as a delayed immune-related event after discontinuation of nivolumab: A case report.Medicine (Baltimore). 2022 Sep 2;101(35):e30456. doi: 10.1097/MD.0000000000030456. Medicine (Baltimore). 2022. PMID: 36107574 Free PMC article.
-
Sintilimab induced diabetic ketoacidosis in a patient with small cell lung cancer: A case report and literature review.Medicine (Baltimore). 2021 May 14;100(19):e25795. doi: 10.1097/MD.0000000000025795. Medicine (Baltimore). 2021. PMID: 34106616 Free PMC article. Review.
Cited by
-
Turning Points in Cross-Disciplinary Perspective of Primary Hyperparathyroidism and Pancreas Involvements: Hypercalcemia-Induced Pancreatitis, MEN1 Gene-Related Tumors, and Insulin Resistance.Int J Mol Sci. 2024 Jun 8;25(12):6349. doi: 10.3390/ijms25126349. Int J Mol Sci. 2024. PMID: 38928056 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

