Efficacy and Safety of Anti-CGRP Monoclonal Antibodies in Prevention of Chronic Migraine: A Bayesian Network Meta-analysis
- PMID: 38247409
- PMCID: PMC10811399
- DOI: 10.9758/cpn.23.1109
Efficacy and Safety of Anti-CGRP Monoclonal Antibodies in Prevention of Chronic Migraine: A Bayesian Network Meta-analysis
Abstract
Due to the unmet needs in the management of migraine, a primary headache, and disabling disorder, the past decade has focused on developing monoclonal antibodies (mAbs) against the calcitonin-gene-related peptide (CGRP) as migraine prophylactic agents. The objective of the study was to evaluate the efficacy and safety of various anti-CGRP mAbs in the prevention of chronic migraine. Network meta-analysis (NMA) was performed using the Bayesian framework to estimate the efficacy and safety of mAbs after performing a literature search in PubMed, MEDLINE, Cochrane database, and International Clinical Trial Registry Platform (ICTRP). The outcomes calculated were in terms of mean difference (MD) or odds ratio (OR) with a 95% credible interval (95%CrI). Network graphs were constructed and node-split analysis was done to analyze the inconsistency. The NMA included a total of 10 clinical trials. Galacanezumab (120 mg) (MD: -2.7; 95%CrI: -4.8 to -0.83) was found to be better than other mAbs in terms of the difference in mean migraine days (MMD). Fremanezumab quarterly dose administration showed the best response (OR: 2.9; 95%CrI: 1.9 to 4.6) in terms of responder rate. Eptinezumab was found to be safer (OR: 0.88; 95%CrI: 0.61-1.3) as compared to other mAbs in terms of the rate of adverse events. Fremanezumab (quarterly) ranked better in terms of response rate, and eptinezumab was found to be the safest in the prophylactic management of migraine. Galacenequmab was better at reducing MMD. Further studies are needed to evaluate the long-term safety, efficacy, and use of mAbs in migraine patients.
Keywords: Antibodies; Calcitonin-gene-related peptide; Migraine; Network meta-analysis; monoclonal.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
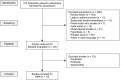
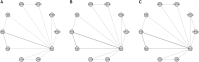


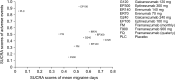
References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Col-laborators, author. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

