T3 Intratracheal Therapy Alleviates Pulmonary Pathology in an Elastase-Induced Emphysema-Dominant COPD Mouse Model
- PMID: 38247455
- PMCID: PMC10812479
- DOI: 10.3390/antiox13010030
T3 Intratracheal Therapy Alleviates Pulmonary Pathology in an Elastase-Induced Emphysema-Dominant COPD Mouse Model
Abstract
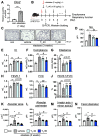
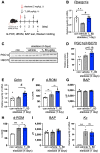
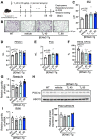
Chronic obstructive pulmonary disease (COPD) is a complex pulmonary condition characterized by bronchitis, emphysema, and mucus stasis. Due to the variability in symptoms among patients, traditional approaches to treating COPD as a singular disease are limited. This led us to focus on phenotype/endotype classifications. In this study, we explore the potential therapeutic role of thyroid hormone (T3) by using mouse models: emphysema-dominant elastase-induced COPD and airway-dominant C57BL/6-βENaC-Tg to represent different types of the disease. Here, we showed that intratracheal T3 treatment (40, 80 μg/kg, i.t., every other day) resulted in significant improvements regarding emphysema and the enhancement of respiratory function in the elastase-induced COPD model. T3-dependent improvement is likely linked to the up-regulation of Ppargc1a, a master regulator of mitochondrial biogenesis, and Gclm, a factor associated with oxidative stress. Conversely, neither short- nor long-term T3 treatments improved COPD pathology in the C57BL/6-βENaC-Tg mice. Because the up-regulation of extrathyroidal T3-producing enzyme Dio2, which is also considered a marker of T3 requirement, was specifically observed in elastase-induced COPD lungs, these results demonstrate that exogenous T3 supplementation may have therapeutic potential for acute but not chronic COPD exacerbation. Moreover, this study highlights the relevance of considering not only COPD phenotypes but also COPD endotypes (expression levels of Ppargc1a and/or Dio2) in the research and development of better treatment approaches for COPD.
Keywords: antioxidant effect; chronic obstructive pulmonary disease (COPD); disease type classification; mitochondrial function; pulmo-modulatory factors; thyroid hormone (T3).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

