Vitamin B12 Supplementation Improves Oocyte Development by Modulating Mitochondria and Yolk Protein in a Caffeine-Ingested Caenorhabditis elegans Model
- PMID: 38247478
- PMCID: PMC10812423
- DOI: 10.3390/antiox13010053
Vitamin B12 Supplementation Improves Oocyte Development by Modulating Mitochondria and Yolk Protein in a Caffeine-Ingested Caenorhabditis elegans Model
Abstract
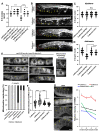
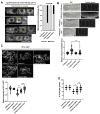
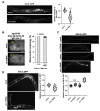
Vitamin B12 is an essential cofactor involved in the function of two enzymes: cytosolic methionine synthase and mitochondrial methylmalonic-CoA mutase. In our previous studies, caffeine (1,3,7-trimethylxanthine), the most popular bioactivator, was shown to reduce yolk protein (vitellogenin) and fertility in a Caenorhabditis elegans model. Based on the previous finding that methionine supplementation increases vitellogenesis in C. elegans, we investigated the role of vitamin B12 in methionine-mediated vitellogenesis during oogenesis in caffeine-ingested animals (CIA). Vitamin B12 supplementation improved vitellogenesis and reduced oxidative stress by decreasing mitochondrial function in CIA. Furthermore, the decreased number of developing oocytes and high levels of reactive oxygen species in oocytes from CIA were recovered with vitamin B12 supplementation through a reduction in mitochondrial stress, which increased vitellogenesis. Taken together, vitamin B12 supplementation can reverse the negative effects of caffeine intake by enhancing methionine-mediated vitellogenesis and oocyte development by reducing mitochondrial stress.
Keywords: Caenorhabditis elegans; caffeine; mitochondria; oogenesis; vitamin B12; yolk protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Methionine Supplementation Alleviates the Germ Cell Apoptosis Increased by Maternal Caffeine Intake in a C. elegans Model.Nutrients. 2024 Mar 20;16(6):894. doi: 10.3390/nu16060894. Nutrients. 2024. PMID: 38542805 Free PMC article.
-
Maternal Caffeine Intake Disrupts Eggshell Integrity and Retards Larval Development by Reducing Yolk Production in a Caenorhabditis elegans Model.Nutrients. 2020 May 7;12(5):1334. doi: 10.3390/nu12051334. Nutrients. 2020. PMID: 32392893 Free PMC article.
-
Long-Term Caffeine Intake Exerts Protective Effects on Intestinal Aging by Regulating Vitellogenesis and Mitochondrial Function in an Aged Caenorhabditis Elegans Model.Nutrients. 2021 Jul 23;13(8):2517. doi: 10.3390/nu13082517. Nutrients. 2021. PMID: 34444677 Free PMC article.
-
Biochemistry, function, and deficiency of vitamin B12 in Caenorhabditis elegans.Exp Biol Med (Maywood). 2016 Sep;241(15):1663-8. doi: 10.1177/1535370216662713. Epub 2016 Aug 2. Exp Biol Med (Maywood). 2016. PMID: 27486161 Free PMC article. Review.
-
Vitamin B12-Multifaceted In Vivo Functions and In Vitro Applications.Nutrients. 2023 Jun 13;15(12):2734. doi: 10.3390/nu15122734. Nutrients. 2023. PMID: 37375638 Free PMC article. Review.
Cited by
-
Caffeine-Induced Upregulation of pas-1 and pas-3 Enhances Intestinal Integrity by Reducing Vitellogenin in Aged Caenorhabditis elegans Model.Nutrients. 2024 Dec 12;16(24):4298. doi: 10.3390/nu16244298. Nutrients. 2024. PMID: 39770921 Free PMC article.
-
Methionine Supplementation Alleviates the Germ Cell Apoptosis Increased by Maternal Caffeine Intake in a C. elegans Model.Nutrients. 2024 Mar 20;16(6):894. doi: 10.3390/nu16060894. Nutrients. 2024. PMID: 38542805 Free PMC article.
References
-
- Haraguchi A., Yamazaki T., Ryan C., Ito K., Sato S., Tamura K., Sekiguchi M., Cao S., Shibata S. Caffeine Suppresses High-Fat Diet-Induced Body Weight Gain in Mice Depending on Feeding Timing. J. Funct. Foods. 2022;99:105307. doi: 10.1016/j.jff.2022.105307. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

