A Prunus avium L. Infusion Inhibits Sugar Uptake and Counteracts Oxidative Stress-Induced Stimulation of Glucose Uptake by Intestinal Epithelial (Caco-2) Cells
- PMID: 38247483
- PMCID: PMC10812648
- DOI: 10.3390/antiox13010059
A Prunus avium L. Infusion Inhibits Sugar Uptake and Counteracts Oxidative Stress-Induced Stimulation of Glucose Uptake by Intestinal Epithelial (Caco-2) Cells
Abstract
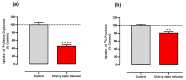
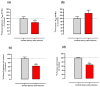
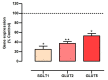
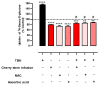
Sweet cherry (Prunus avium L.) is among the most valued fruits due to its organoleptic properties and nutritional worth. Cherry stems are rich in bioactive compounds, known for their anti-inflammatory and antioxidant properties. Innumerable studies have indicated that some bioactive compounds can modulate sugar absorption in the small intestine. In this study, the phenolic profile of a cherry stem infusion was investigated, as well as its capacity to modulate intestinal glucose and fructose transport in Caco-2 cells. Long-term (24 h) exposure to cherry stem infusion (25%, v/v) significantly reduced glucose (3H-DG) and fructose (14C-FRU) apical uptake, reduced the apical-to-basolateral Papp to 3H-DG, and decreased mRNA expression levels of the sugar transporters SGLT1, GLUT2 and GLUT5. Oxidative stress (induced by tert-butyl hydroperoxide) caused an increase in 3H-DG uptake, which was abolished by the cherry stem infusion. These findings suggest that cherry stem infusion can reduce the intestinal absorption of both glucose and fructose by decreasing the gene expression of their membrane transporters. Moreover, this infusion also appears to be able to counteract the stimulatory effect of oxidative stress upon glucose intestinal uptake. Therefore, it can be a potentially useful compound for controlling hyperglycemia, especially in the presence of increased intestinal oxidative stress levels.
Keywords: antioxidant activity; cherry stem; infusion; intestinal sugar uptake; phenolic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The effect of oxidative stress upon intestinal sugar transport: an in vitro study using human intestinal epithelial (Caco-2) cells.Toxicol Res (Camb). 2018 Sep 17;7(6):1236-1246. doi: 10.1039/c8tx00183a. eCollection 2018 Nov 1. Toxicol Res (Camb). 2018. PMID: 30542607 Free PMC article.
-
TNFα regulates sugar transporters in the human intestinal epithelial cell line Caco-2.Cytokine. 2013 Oct;64(1):181-7. doi: 10.1016/j.cyto.2013.07.004. Epub 2013 Jul 31. Cytokine. 2013. PMID: 23910014
-
Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice.J Physiol. 2003 Nov 1;552(Pt 3):823-32. doi: 10.1113/jphysiol.2003.049247. Epub 2003 Aug 22. J Physiol. 2003. PMID: 12937289 Free PMC article.
-
Glucose transporters in the small intestine in health and disease.Pflugers Arch. 2020 Sep;472(9):1207-1248. doi: 10.1007/s00424-020-02439-5. Epub 2020 Aug 23. Pflugers Arch. 2020. PMID: 32829466 Free PMC article. Review.
-
Does apical membrane GLUT2 have a role in intestinal glucose uptake?F1000Res. 2014 Dec 12;3:304. doi: 10.12688/f1000research.5934.1. eCollection 2014. F1000Res. 2014. PMID: 25671087 Free PMC article. Review.
Cited by
-
Association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and cognitive impairment in patients with acute mild ischemic stroke.Eur J Med Res. 2025 May 30;30(1):430. doi: 10.1186/s40001-025-02693-2. Eur J Med Res. 2025. PMID: 40448222 Free PMC article.
-
D-Glucose and its derivatives labeled with radioactive carbon and hydrogen: key tools for investigating biological processes and molecular mechanisms.EJNMMI Radiopharm Chem. 2025 May 30;10(1):27. doi: 10.1186/s41181-025-00346-7. EJNMMI Radiopharm Chem. 2025. PMID: 40445480 Free PMC article. Review.
-
Suitability of red fox (Vulpes vulpes) and golden jackal (Canis aureus) as hosts of Echinococcus multilocularis based on egg production characteristics and literature data on the intestinal ecosystems of carnivores.Curr Res Parasitol Vector Borne Dis. 2024 Oct 28;6:100225. doi: 10.1016/j.crpvbd.2024.100225. eCollection 2024. Curr Res Parasitol Vector Borne Dis. 2024. PMID: 39554486 Free PMC article.
References
-
- Rahman M.M., Rahaman M.S., Islam M.R., Rahman F., Mithi F.M., Alqahtani T., Almikhlafi M.A., Alghamdi S.Q., Alruwaili A.S., Hossain M.S., et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules. 2021;27:233. doi: 10.3390/molecules27010233. - DOI - PMC - PubMed
-
- Nunes A.R., Gonçalves A.C., Falcão A., Alves G., Silva L.R. Prunus avium L. (Sweet Cherry) By-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021;11:8516. doi: 10.3390/app11188516. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

