Amphiregulin Exerts Proangiogenic Effects in Developing Murine Lungs
- PMID: 38247502
- PMCID: PMC10812697
- DOI: 10.3390/antiox13010078
Amphiregulin Exerts Proangiogenic Effects in Developing Murine Lungs
Abstract
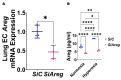
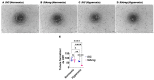
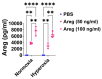
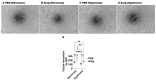
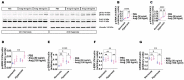
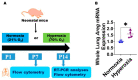
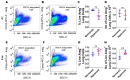
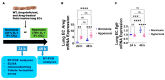
Interrupted lung angiogenesis is a hallmark of bronchopulmonary dysplasia (BPD); however, druggable targets that can rescue this phenotype remain elusive. Thus, our investigation focused on amphiregulin (Areg), a growth factor that mediates cellular proliferation, differentiation, migration, survival, and repair. While Areg promotes lung branching morphogenesis, its effect on endothelial cell (EC) homeostasis in developing lungs is understudied. Therefore, we hypothesized that Areg promotes the proangiogenic ability of the ECs in developing murine lungs exposed to hyperoxia. Lung tissues were harvested from neonatal mice exposed to normoxia or hyperoxia to determine Areg expression. Next, we performed genetic loss-of-function and pharmacological gain-of-function studies in normoxia- and hyperoxia-exposed fetal murine lung ECs. Hyperoxia increased Areg mRNA levels and Areg+ cells in whole lungs. While Areg expression was increased in lung ECs exposed to hyperoxia, the expression of its signaling receptor, epidermal growth factor receptor, was decreased, indicating that hyperoxia reduces Areg signaling in lung ECs. Areg deficiency potentiated hyperoxia-mediated anti-angiogenic effects. In contrast, Areg treatment increased extracellular signal-regulated kinase activation and exerted proangiogenic effects. In conclusion, Areg promotes EC tubule formation in developing murine lungs exposed to hyperoxia.
Keywords: amphiregulin; angiogenesis; bronchopulmonary dysplasia; fetal murine lung endothelial cells; hyperoxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Tie-2 Cre-Mediated Deficiency of Extracellular Signal-Regulated Kinase 2 Potentiates Experimental Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension in Neonatal Mice.Int J Mol Sci. 2020 Mar 31;21(7):2408. doi: 10.3390/ijms21072408. Int J Mol Sci. 2020. PMID: 32244398 Free PMC article.
-
Type 2 innate lymphoid cell-derived amphiregulin regulates type II alveolar epithelial cell transdifferentiation in a mouse model of bronchopulmonary dysplasia.Int Immunopharmacol. 2023 Sep;122:110672. doi: 10.1016/j.intimp.2023.110672. Epub 2023 Jul 20. Int Immunopharmacol. 2023. PMID: 37480752
-
Endothelial Adenosine Monophosphate-Activated Protein Kinase-Alpha1 Deficiency Potentiates Hyperoxia-Induced Experimental Bronchopulmonary Dysplasia and Pulmonary Hypertension.Antioxidants (Basel). 2021 Nov 29;10(12):1913. doi: 10.3390/antiox10121913. Antioxidants (Basel). 2021. PMID: 34943016 Free PMC article.
-
The Role of Sphingolipid Signaling in Oxidative Lung Injury and Pathogenesis of Bronchopulmonary Dysplasia.Int J Mol Sci. 2022 Jan 23;23(3):1254. doi: 10.3390/ijms23031254. Int J Mol Sci. 2022. PMID: 35163176 Free PMC article. Review.
-
Amphiregulin in infectious diseases: Role, mechanism, and potential therapeutic targets.Microb Pathog. 2024 Jan;186:106463. doi: 10.1016/j.micpath.2023.106463. Epub 2023 Nov 28. Microb Pathog. 2024. PMID: 38036111 Review.
Cited by
-
Emerging Roles of Eosinophils in Bone.Curr Osteoporos Rep. 2025 Apr 4;23(1):17. doi: 10.1007/s11914-025-00913-6. Curr Osteoporos Rep. 2025. PMID: 40183859 Free PMC article. Review.
References
-
- Twilhaar E.S., Wade R.M., de Kieviet J.F., van Goudoever J.B., van Elburg R.M., Oosterlaan J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr. 2018;172:361–367. doi: 10.1001/jamapediatrics.2017.5323. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

