Hypoxia-Inducible Factor and Oxidative Stress in Tendon Degeneration: A Molecular Perspective
- PMID: 38247510
- PMCID: PMC10812560
- DOI: 10.3390/antiox13010086
Hypoxia-Inducible Factor and Oxidative Stress in Tendon Degeneration: A Molecular Perspective
Abstract
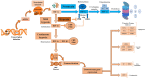
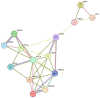
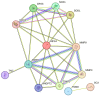
Tendinopathy is a debilitating condition marked by degenerative changes in the tendons. Its complex pathophysiology involves intrinsic, extrinsic, and physiological factors. While its intrinsic and extrinsic factors have been extensively studied, the role of physiological factors, such as hypoxia and oxidative stress, remains largely unexplored. This review article delves into the contribution of hypoxia-associated genes and oxidative-stress-related factors to tendon degeneration, offering insights into potential therapeutic strategies. The unique aspect of this study lies in its pathway-based evidence, which sheds light on how these factors can be targeted to enhance overall tendon health.
Keywords: degenerative disorders; hypoxia; oxidative stress; tendinopathy; tendon disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bordoni B., Anatomy V.M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Tendons.
-
- de Cassia Marqueti R., de Sousa Neto I.V., Barin F.R., Ramos G.V. Tendons. IntechOpen; Rijeka, Croatia: 2019. Exercise and Tendon Remodeling Mechanism. - DOI
-
- Tran P.H.T., Malmgaard-Clausen N.M., Puggaard R.S., Svensson R.B., Nybing J.D., Hansen P., Schjerling P., Zinglersen A.H., Couppé C., Boesen M., et al. Early development of tendinopathy in humans: Sequence of pathological changes in structure and tissue turnover signaling. FASEB J. 2020;34:776–788. doi: 10.1096/fj.201901309R. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

