Diabetic Keratopathy: Redox Signaling Pathways and Therapeutic Prospects
- PMID: 38247544
- PMCID: PMC10812573
- DOI: 10.3390/antiox13010120
Diabetic Keratopathy: Redox Signaling Pathways and Therapeutic Prospects
Abstract
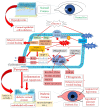
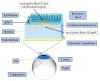
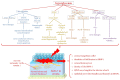
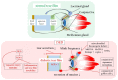
Diabetes mellitus, the most prevalent endocrine disorder, not only impacts the retina but also significantly involves the ocular surface. Diabetes contributes to the development of dry eye disease and induces morphological and functional corneal alterations, particularly affecting nerves and epithelial cells. These changes manifest as epithelial defects, reduced sensitivity, and delayed wound healing, collectively encapsulated in the context of diabetic keratopathy. In advanced stages of this condition, the progression to corneal ulcers and scarring further unfolds, eventually leading to corneal opacities. This critical complication hampers vision and carries the potential for irreversible visual loss. The primary objective of this review article is to offer a comprehensive overview of the pathomechanisms underlying diabetic keratopathy. Emphasis is placed on exploring the redox molecular pathways responsible for the aberrant structural changes observed in the cornea and tear film during diabetes. Additionally, we provide insights into the latest experimental findings concerning potential treatments targeting oxidative stress. This endeavor aims to enhance our understanding of the intricate interplay between diabetes and ocular complications, offering valuable perspectives for future therapeutic interventions.
Keywords: cornea; diabetic complication; keratopathy; molecular; pathways; redox; targets.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
-
- Seifart U., Strempel I. The dry eye and diabetes mellitus. Ophthalmologe. 1994;91:235–239. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

