Clinical and Analytical Performance of ELISA Salivary Serologic Assay to Detect SARS-CoV-2 IgG in Children and Adults
- PMID: 38247570
- PMCID: PMC10801479
- DOI: 10.3390/antib13010006
Clinical and Analytical Performance of ELISA Salivary Serologic Assay to Detect SARS-CoV-2 IgG in Children and Adults
Abstract
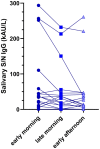
Saliva is a promising matrix with several purposes. Our aim is to verify if salivary anti-SARS-CoV-2 antibody determination is suitable for monitoring immune responses. One hundred eighty-seven subjects were enrolled at University-Hospital Padova: 105 females (56.1%) and 82 males (43.9%), 95 (50.8%) children and 92 (49.2%) adults. Subjects self-collected saliva using Salivette; nineteen subjects collected three different samples within the day. A serum sample was obtained for all individuals. The N/S anti-SARS-CoV-2 salivary IgG (sal-IgG) and serum anti-SARS-CoV-2 S-RBD IgG (ser-IgG) were used for determining anti-SARS-CoV-2 antibodies. The mean (min-max) age was 9.0 (1-18) for children and 42.5 (20-61) for adults. Of 187 samples, 63 were negative for sal-IgG (33.7%), while 7 were negative for ser-IgG (3.7%). Spearman's correlation was 0.56 (p < 0.001). Sal-IgG and ser-IgG levels were correlated with age but not with gender, comorbidities, prolonged therapy, previous SARS-CoV-2 infection, or time from last COVID-19 infection/vaccination. The repeatability ranged from 23.8% (7.4 kAU/L) to 4.0% (3.77 kAU/L). The linearity of the assay was missed in 4/6 samples. No significant intrasubject differences were observed in sal-IgG across samples collected at different time points. Sal-IgG has good agreement with ser-IgG. Noninvasive saliva collection represents an alternative method for antibody measurement, especially in children.
Keywords: ELISA; SARS-CoV-2 antibodies; adults; children; salivary samples.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Methodological approaches to optimize multiplex oral fluid SARS-CoV-2 IgG assay performance and correlation with serologic and neutralizing antibody responses.J Immunol Methods. 2023 Mar;514:113440. doi: 10.1016/j.jim.2023.113440. Epub 2023 Feb 10. J Immunol Methods. 2023. PMID: 36773929 Free PMC article.
-
Methodological approaches to optimize multiplex oral fluid SARS-CoV-2 IgG assay performance and correlation with serologic and neutralizing antibody responses.medRxiv [Preprint]. 2022 Dec 26:2022.12.22.22283858. doi: 10.1101/2022.12.22.22283858. medRxiv. 2022. Update in: J Immunol Methods. 2023 Mar;514:113440. doi: 10.1016/j.jim.2023.113440. PMID: 36597525 Free PMC article. Updated. Preprint.
-
Salivary and serum IgA and IgG responses to SARS-CoV-2-spike protein following SARS-CoV-2 infection and after immunization with COVID-19 vaccines.Allergy Asthma Proc. 2022 Sep 1;43(5):419-430. doi: 10.2500/aap.2022.43.220045. Allergy Asthma Proc. 2022. PMID: 36065108 Free PMC article.
-
Multiplex Antibody Analysis of IgM, IgA and IgG to SARS-CoV-2 in Saliva and Serum From Infected Children and Their Close Contacts.Front Immunol. 2022 Jan 27;13:751705. doi: 10.3389/fimmu.2022.751705. eCollection 2022. Front Immunol. 2022. PMID: 35154094 Free PMC article.
-
Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review.Front Immunol. 2022 Sep 20;13:1006040. doi: 10.3389/fimmu.2022.1006040. eCollection 2022. Front Immunol. 2022. PMID: 36203571 Free PMC article.
Cited by
-
COVID-19 point-of-care tests can identify low-antibody individuals: In-depth immunoanalysis of boosting benefits in a healthy cohort.Sci Adv. 2024 Jun 14;10(24):eadi1379. doi: 10.1126/sciadv.adi1379. Epub 2024 Jun 12. Sci Adv. 2024. PMID: 38865463 Free PMC article.
-
The omicron variant of SARS-CoV-2 drove broadly increased seroprevalence in a public university setting.PLOS Glob Public Health. 2025 Jan 3;5(1):e0003893. doi: 10.1371/journal.pgph.0003893. eCollection 2025. PLOS Glob Public Health. 2025. PMID: 39752417 Free PMC article.
References
-
- Statement on the Fourteenth Meeting of the International Health Regulations (2005) Emergency Committee regarding the Coronavirus Disease (COVID-19) Pandemic. World Health Organization (WHO); Geneva, Switzerland: 2023.
-
- Cosma C., Galla L., Padoan A., Furlan G., Marchioro L., Zaninotto M., Basso D., Plebani M. SARS-CoV-2 specific T-cell humoral response assessment after COVID-19 vaccination using a rapid direct real-time PCR amplification. Clin. Chem. Lab. Med. (CCLM) 2023;61:1652–1660. doi: 10.1515/cclm-2023-0129. - DOI - PubMed
-
- Padoan A., Cosma C., Bonfante F., Della Rocca F., Barbaro F., Santarossa C., DallOlmo L., Pagliari M., Bortolami A., Cattelan A., et al. SARS-CoV-2 neutralizing antibodies after one or two doses of Comirnaty (BNT162b2 BioNTech/Pfizer): Kinetics and comparison with chemiluminescent assays. Clin. Chim. Acta. 2021;523:446–453. doi: 10.1016/j.cca.2021.10.028. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

