Drug Discovery in the Field of β-Lactams: An Academic Perspective
- PMID: 38247618
- PMCID: PMC10812508
- DOI: 10.3390/antibiotics13010059
Drug Discovery in the Field of β-Lactams: An Academic Perspective
Abstract
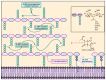
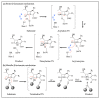
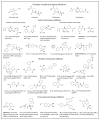
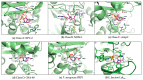
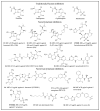
β-Lactams are the most widely prescribed class of antibiotics that inhibit penicillin-binding proteins (PBPs), particularly transpeptidases that function in peptidoglycan synthesis. A major mechanism of antibiotic resistance is the production of β-lactamase enzymes, which are capable of hydrolyzing β-lactam antibiotics. There have been many efforts to counter increasing bacterial resistance against β-lactams. These studies have mainly focused on three areas: discovering novel inhibitors against β-lactamases, developing new β-lactams less susceptible to existing resistance mechanisms, and identifying non-β-lactam inhibitors against cell wall transpeptidases. Drug discovery in the β-lactam field has afforded a range of research opportunities for academia. In this review, we summarize the recent new findings on both β-lactamases and cell wall transpeptidases because these two groups of enzymes are evolutionarily and functionally connected. Many efforts to develop new β-lactams have aimed to inhibit both transpeptidases and β-lactamases, while several promising novel β-lactamase inhibitors have shown the potential to be further developed into transpeptidase inhibitors. In addition, the drug discovery progress against each group of enzymes is presented in three aspects: understanding the targets, screening methodology, and new inhibitor chemotypes. This is to offer insights into not only the advancement in this field but also the challenges, opportunities, and resources for future research. In particular, cyclic boronate compounds are now capable of inhibiting all classes of β-lactamases, while the diazabicyclooctane (DBO) series of small molecules has led to not only new β-lactamase inhibitors but potentially a new class of antibiotics by directly targeting PBPs. With the cautiously optimistic successes of a number of new β-lactamase inhibitor chemotypes and many questions remaining to be answered about the structure and function of cell wall transpeptidases, non-β-lactam transpeptidase inhibitors may usher in the next exciting phase of drug discovery in this field.
Keywords: cell wall transpeptidase; peptidoglycan; resistance mechanisms; β-lactam; β-lactamase; β-lactamase inhibitor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

