Nuclear Factor-κB Decoy Oligodeoxynucleotide Attenuates Cartilage Resorption In Vitro
- PMID: 38247922
- PMCID: PMC10813736
- DOI: 10.3390/bioengineering11010046
Nuclear Factor-κB Decoy Oligodeoxynucleotide Attenuates Cartilage Resorption In Vitro
Abstract
Background: Cartilage harvest and transplantation is a common surgery using costal, auricular, and septal cartilage for craniofacial reconstruction. However, absorption and warping of the cartilage grafts can occur due to inflammatory factors associated with wound healing. Transcription factor nuclear factor-κB (NF-κB) is activated by the various stimulation such as interleukin-1 (IL-1), and plays a central role in the transactivation of this inflammatory cytokine gene. Inhibition of NF-κB may have anti-inflammatory effects. The aim of this study was to explore the potential of an NF-κB decoy oligodeoxynucleotide (Decoy) as a chondroprotective agent.
Materials and methods: Safe and efficacious concentrations of Decoy were assessed using rabbit nasal septal chondrocytes (rNSChs) and assays for cytotoxicity, proteoglycan (PG) synthesis, and PG turnover were carried out. The efficacious concentration of Decoy determined from the rNSChs was then applied to human nasal septal cartilage (hNSC) in vitro and analyzed for PG turnover, the levels of inflammatory markers, and catabolic enzymes in explant-conditioned culture medium.
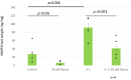
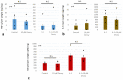
Results: Over the range of Decoy conditions and concentrations, no inhibition of PG synthesis or cytotoxicity was observed. Decoy at 10 μM effectively inhibited PG degradation in the hNSC explant, prolonging PG half-life by 63% and decreasing matrix metalloprotease 3 (MMP-3) by 70.7% (p = 0.027).
Conclusions: Decoy may be considered a novel chondroprotective therapeutic agent in cartilage transplantation due to its ability to inhibit cartilage degradation due to inflammation cytokines.
Keywords: cartilage; decoy oligodeoxynucleotide; nuclear factor-κb; resorption.
Conflict of interest statement
K.M. has received research non-related grants from AnGes inc. K.M. and D.S. have been involved as consultants in AnGes Inc. for different applications. H.N. and D.W. have no conflicts of interest.
Figures






Similar articles
-
Mori folium inhibits interleukin-1β-induced expression of matrix metalloproteinases and inflammatory mediators by suppressing the activation of NF-κB and p38 MAPK in SW1353 human chondrocytes.Int J Mol Med. 2016 Feb;37(2):452-60. doi: 10.3892/ijmm.2015.2443. Epub 2015 Dec 23. Int J Mol Med. 2016. PMID: 26707272
-
Inhibition of cartilage degradation and suppression of PGE2 and MMPs expression by pomegranate fruit extract in a model of posttraumatic osteoarthritis.Nutrition. 2017 Jan;33:1-13. doi: 10.1016/j.nut.2016.08.004. Epub 2016 Sep 2. Nutrition. 2017. PMID: 27908544 Free PMC article.
-
NF-κB Decoy ODN-Loaded Poly(Lactic-co-glycolic Acid) Nanospheres Inhibit Alveolar Ridge Resorption.Int J Mol Sci. 2023 Feb 12;24(4):3699. doi: 10.3390/ijms24043699. Int J Mol Sci. 2023. PMID: 36835111 Free PMC article.
-
Prevention of Asthma Exacerbation in a Mouse Model by Simultaneous Inhibition of NF-κB and STAT6 Activation Using a Chimeric Decoy Strategy.Mol Ther Nucleic Acids. 2018 Mar 2;10:159-169. doi: 10.1016/j.omtn.2017.12.005. Epub 2017 Dec 14. Mol Ther Nucleic Acids. 2018. PMID: 29499930 Free PMC article.
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
References
-
- De Gabory L., Fricain J.C., Stoll D. Resorption of cartilage grafts in rhinoplasty: Fundamental basis. Rev. Laryngol.-Otol.-Rhinol. 2010;131:83–88. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

