Osteoblast Attachment on Bioactive Glass Air Particle Abrasion-Induced Calcium Phosphate Coating
- PMID: 38247951
- PMCID: PMC10813256
- DOI: 10.3390/bioengineering11010074
Osteoblast Attachment on Bioactive Glass Air Particle Abrasion-Induced Calcium Phosphate Coating
Abstract
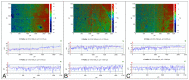
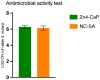
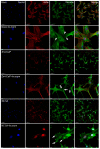
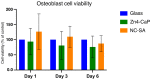
Air particle abrasion (APA) using bioactive glass (BG) effectively decontaminates titanium (Ti) surface biofilms and the retained glass particles on the abraded surfaces impart potent antibacterial properties against various clinically significant pathogens. The objective of this study was to investigate the effect of BG APA and simulated body fluid (SBF) immersion of sandblasted and acid-etched (SA) Ti surfaces on osteoblast cell viability. Another goal was to study the antibacterial effect against Streptococcus mutans. Square-shaped 10 mm diameter Ti substrates (n = 136) were SA by grit blasting with aluminum oxide particles, then acid-etching in an HCl-H2SO4 mixture. The SA substrates (n = 68) were used as non-coated controls (NC-SA). The test group (n = 68) was further subjected to APA using experimental zinc-containing BG (Zn4) and then mineralized in SBF for 14 d (Zn4-CaP). Surface roughness, contact angle, and surface free energy (SFE) were calculated on test and control surfaces. In addition, the topography and chemistry of substrate surfaces were also characterized. Osteoblastic cell viability and focal adhesion were also evaluated and compared to glass slides as an additional control. The antibacterial effect of Zn4-CaP was also assessed against S. mutans. After immersion in SBF, a mineralized zinc-containing Ca-P coating was formed on the SA substrates. The Zn4-CaP coating resulted in a significantly lower Ra surface roughness value (2.565 μm; p < 0.001), higher wettability (13.35°; p < 0.001), and higher total SFE (71.13; p < 0.001) compared to 3.695 μm, 77.19° and 40.43 for the NC-SA, respectively. APA using Zn4 can produce a zinc-containing calcium phosphate coating that demonstrates osteoblast cell viability and focal adhesion comparable to that on NC-SA or glass slides. Nevertheless, the coating had no antibacterial effect against S. mutans.
Keywords: S. mutans; biofilm; biomineralization; implant; osteoblast; peri-implantitis; titanium; zinc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Exploring the Reactions Induced by Bioactive Glass Air Abrasion of Titanium and Their Effects on Osteoblast Cellular Responses.J Biomed Mater Res A. 2025 Jun;113(6):e37949. doi: 10.1002/jbm.a.37949. J Biomed Mater Res A. 2025. PMID: 40522065
-
Air Abrasion With Bioactive Glass Eradicates Streptococcus mutans Biofilm From a Sandblasted and Acid-Etched Titanium Surface.J Oral Implantol. 2019 Dec;45(6):444-450. doi: 10.1563/aaid-joi-D-18-00324. Epub 2019 Sep 19. J Oral Implantol. 2019. PMID: 31536440
-
Effect of bioactive glass air-abrasion on the wettability and osteoblast proliferation on sandblasted and acid-etched titanium surfaces.Eur J Oral Sci. 2020 Apr;128(2):160-169. doi: 10.1111/eos.12683. Epub 2020 Mar 10. Eur J Oral Sci. 2020. PMID: 32154611
-
Effect of bioactive glass air-abrasion on Fusobacterium nucleatum and Porphyromonas gingivalis biofilm formed on moderately rough titanium surface.Eur J Oral Sci. 2021 Jun;129(3):e12783. doi: 10.1111/eos.12783. Epub 2021 Mar 16. Eur J Oral Sci. 2021. PMID: 33724569
-
Bioactive glass air-abrasion promotes healing around contaminated implant surfaces surrounded by circumferential bone defects: An experimental study in the rat.Clin Implant Dent Relat Res. 2023 Apr;25(2):409-418. doi: 10.1111/cid.13172. Epub 2023 Jan 5. Clin Implant Dent Relat Res. 2023. PMID: 36602418
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

