Management of Cytomegalovirus Infections in the Era of the Novel Antiviral Players, Letermovir and Maribavir
- PMID: 38247977
- PMCID: PMC10801527
- DOI: 10.3390/idr16010005
Management of Cytomegalovirus Infections in the Era of the Novel Antiviral Players, Letermovir and Maribavir
Abstract
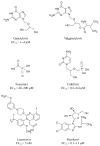
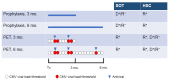
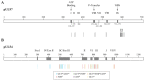
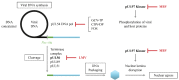
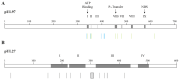
Cytomegalovirus (CMV) infections may increase morbidity and mortality in immunocompromised patients. Until recently, standard antiviral drugs against CMV were limited to viral DNA polymerase inhibitors (val)ganciclovir, foscarnet and cidofovir with a risk for cross-resistance. These drugs may also cause serious side effects. This narrative review provides an update on new antiviral agents that were approved for the prevention and treatment of CMV infections in transplant recipients. Letermovir was approved in 2017 for CMV prophylaxis in CMV-seropositive adults who received an allogeneic hematopoietic stem cell transplant. Maribavir followed four years later, with an indication in the treatment of adult and pediatric transplant patients with refractory/resistant CMV disease. The target of letermovir is the CMV terminase complex (constituted of pUL56, pUL89 and pUL51 subunits). Letermovir prevents the cleavage of viral DNA and its packaging into capsids. Maribavir is a pUL97 kinase inhibitor, which interferes with the assembly of capsids and the egress of virions from the nucleus. Both drugs have activity against most CMV strains resistant to standard drugs and exhibit favorable safety profiles. However, high-level resistance mutations may arise more rapidly in the UL56 gene under letermovir than low-grade resistance mutations. Some mutations emerging in the UL97 gene under maribavir can be cross-resistant with ganciclovir. Thus, letermovir and maribavir now extend the drug arsenal available for the management of CMV infections and their respective niches are currently defined.
Keywords: antiviral drugs; cytomegalovirus; drug resistance; immunocompromised patients; letermovir; maribavir.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance.Antiviral Res. 2019 Mar;163:91-105. doi: 10.1016/j.antiviral.2019.01.011. Epub 2019 Jan 25. Antiviral Res. 2019. PMID: 30690043 Review.
-
Current Perspectives on Letermovir and Maribavir for the Management of Cytomegalovirus Infection in Solid Organ Transplant Recipients.Drug Des Devel Ther. 2024 Sep 6;18:3987-4001. doi: 10.2147/DDDT.S265644. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39258274 Free PMC article. Review.
-
Letermovir for the prevention of cytomegalovirus infection and disease in transplant recipients: an evidence-based review.Infect Drug Resist. 2019 Jun 4;12:1481-1491. doi: 10.2147/IDR.S180908. eCollection 2019. Infect Drug Resist. 2019. PMID: 31239725 Free PMC article. Review.
-
Moving Past Ganciclovir and Foscarnet: Advances in CMV Therapy.Curr Hematol Malig Rep. 2020 Apr;15(2):90-102. doi: 10.1007/s11899-020-00557-6. Curr Hematol Malig Rep. 2020. PMID: 31981100 Free PMC article. Review.
-
Assessment of UL56 Mutations before Letermovir Therapy in Refractory Cytomegalovirus Transplant Recipients.Microbiol Spectr. 2022 Apr 27;10(2):e0019122. doi: 10.1128/spectrum.00191-22. Epub 2022 Mar 28. Microbiol Spectr. 2022. PMID: 35343771 Free PMC article.
Cited by
-
Cytomegalovirus infection and cardiovascular outcomes in abdominal organ transplant recipients: A systematic review and meta-analysis.Transplant Rev (Orlando). 2024 Dec;38(4):100860. doi: 10.1016/j.trre.2024.100860. Epub 2024 May 25. Transplant Rev (Orlando). 2024. PMID: 38815340
-
Tackling CMV in Transplant Recipients: Past, Present, and Future.Infect Dis Ther. 2025 Jun;14(6):1183-1200. doi: 10.1007/s40121-025-01159-6. Epub 2025 Apr 27. Infect Dis Ther. 2025. PMID: 40289195 Free PMC article. Review.
-
Characterization of human cytomegalovirus infection dynamics in human microglia.J Gen Virol. 2025 Apr;106(4):002096. doi: 10.1099/jgv.0.002096. J Gen Virol. 2025. PMID: 40299764 Free PMC article.
-
Refractory/Resistant Cytomegalovirus Infection in Transplant Recipients: An Update.Viruses. 2024 Jul 5;16(7):1085. doi: 10.3390/v16071085. Viruses. 2024. PMID: 39066247 Free PMC article. Review.
-
Cytomegalovirus Retinitis: Clinical Manifestations, Diagnosis and Treatment.Viruses. 2024 Sep 7;16(9):1427. doi: 10.3390/v16091427. Viruses. 2024. PMID: 39339903 Free PMC article. Review.
References
-
- Roizman B., Knipe D.M., Whitley R.J. Herpes simplex viruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Volume 2. Lippincott Williams & Wilkins; Baltimore, MD, USA: 2013. pp. 1823–1897.
-
- Boivin G., Limaye A.P. Goldman-Cecil Medicine. Elsevier; Amsterdam, The Netherlands: 2023. Cytomegalovirus.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

