Clinical Applications of Anterior Segment Optical Coherence Tomography: An Updated Review
- PMID: 38248000
- PMCID: PMC10814678
- DOI: 10.3390/diagnostics14020122
Clinical Applications of Anterior Segment Optical Coherence Tomography: An Updated Review
Abstract
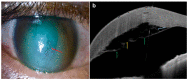
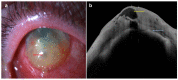
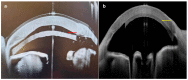
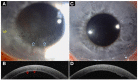
Since its introduction, optical coherence tomography (OCT) has revolutionized the field of ophthalmology and has now become an indispensable, noninvasive tool in daily practice. Most ophthalmologists are familiar with its use in the assessment and monitoring of retinal and optic nerve diseases. However, it also has important applications in the assessment of anterior segment structures, including the cornea, conjunctiva, sclera, anterior chamber, and iris, and has the potential to transform the clinical examination of these structures. In this review, we aim to provide a comprehensive overview of the potential clinical utility of anterior segment OCT (AS-OCT) for a wide range of anterior segment pathologies, such as conjunctival neoplasia, pterygium, scleritis, keratoconus, corneal dystrophies, and infectious/noninfectious keratitis. In addition, the clinical applications of AS-OCT (including epithelial mapping) in preoperative planning and postoperative monitoring for corneal and refractive surgeries are discussed.
Keywords: anterior segment; cornea; epithelial mapping; keratitis; keratoconus; keratoplasty; optical coherence tomography (OCT).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


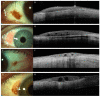
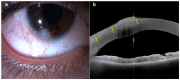

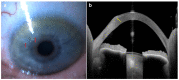
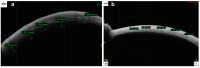




References
-
- Müller P.L., Wolf S., Dolz-Marco R., Tafreshi A., Schmitz-Valckenberg S., Holz F.G. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. Springer; Cham, Switzerland: 2019. Ophthalmic Diagnostic Imaging: Retina; pp. 87–106. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

