Radiomics and Artificial Intelligence in Radiotheranostics: A Review of Applications for Radioligands Targeting Somatostatin Receptors and Prostate-Specific Membrane Antigens
- PMID: 38248059
- PMCID: PMC10814892
- DOI: 10.3390/diagnostics14020181
Radiomics and Artificial Intelligence in Radiotheranostics: A Review of Applications for Radioligands Targeting Somatostatin Receptors and Prostate-Specific Membrane Antigens
Abstract
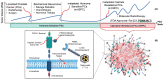
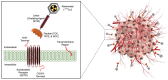
Radiotheranostics refers to the pairing of radioactive imaging biomarkers with radioactive therapeutic compounds that deliver ionizing radiation. Given the introduction of very promising radiopharmaceuticals, the radiotheranostics approach is creating a novel paradigm in personalized, targeted radionuclide therapies (TRTs), also known as radiopharmaceuticals (RPTs). Radiotherapeutic pairs targeting somatostatin receptors (SSTR) and prostate-specific membrane antigens (PSMA) are increasingly being used to diagnose and treat patients with metastatic neuroendocrine tumors (NETs) and prostate cancer. In parallel, radiomics and artificial intelligence (AI), as important areas in quantitative image analysis, are paving the way for significantly enhanced workflows in diagnostic and theranostic fields, from data and image processing to clinical decision support, improving patient selection, personalized treatment strategies, response prediction, and prognostication. Furthermore, AI has the potential for tremendous effectiveness in patient dosimetry which copes with complex and time-consuming tasks in the RPT workflow. The present work provides a comprehensive overview of radiomics and AI application in radiotheranostics, focusing on pairs of SSTR- or PSMA-targeting radioligands, describing the fundamental concepts and specific imaging/treatment features. Our review includes ligands radiolabeled by 68Ga, 18F, 177Lu, 64Cu, 90Y, and 225Ac. Specifically, contributions via radiomics and AI towards improved image acquisition, reconstruction, treatment response, segmentation, restaging, lesion classification, dose prediction, and estimation as well as ongoing developments and future directions are discussed.
Keywords: PSMA; SSTR; artificial intelligence; personalized dosimetry; radiomics; radiotheranostics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Radiotheranostic landscape: A review of clinical and preclinical development.Eur J Nucl Med Mol Imaging. 2025 Jun;52(7):2685-2709. doi: 10.1007/s00259-025-07103-7. Epub 2025 Feb 1. Eur J Nucl Med Mol Imaging. 2025. PMID: 39891713 Review.
-
Clinical Pharmacology of Radiotheranostics in Oncology.Clin Pharmacol Ther. 2023 Feb;113(2):260-274. doi: 10.1002/cpt.2598. Epub 2022 May 10. Clin Pharmacol Ther. 2023. PMID: 35373336 Review.
-
Next generation radiotheranostics promoting precision medicine.Ann Oncol. 2023 Jun;34(6):507-519. doi: 10.1016/j.annonc.2023.03.001. Epub 2023 Mar 15. Ann Oncol. 2023. PMID: 36924989 Review.
-
Somatostatin Receptor Antagonists for Imaging and Therapy.J Nucl Med. 2017 Sep;58(Suppl 2):61S-66S. doi: 10.2967/jnumed.116.186783. J Nucl Med. 2017. PMID: 28864614 Review.
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
Cited by
-
Gastric Emptying Scintigraphy Protocol Optimization Using Machine Learning for the Detection of Delayed Gastric Emptying.Diagnostics (Basel). 2024 Jun 13;14(12):1240. doi: 10.3390/diagnostics14121240. Diagnostics (Basel). 2024. PMID: 38928655 Free PMC article.
-
The role of radiotheranostics in personalized treatment for breast cancer.Med Oncol. 2025 Jul 11;42(8):322. doi: 10.1007/s12032-025-02825-y. Med Oncol. 2025. PMID: 40643742 Review.
-
Automated segmentation of lesions and organs at risk on [68Ga]Ga-PSMA-11 PET/CT images using self-supervised learning with Swin UNETR.Cancer Imaging. 2024 Feb 29;24(1):30. doi: 10.1186/s40644-024-00675-x. Cancer Imaging. 2024. PMID: 38424612 Free PMC article.
References
-
- Teker F., Elboga U. Is SUVmax a useful marker for progression-free survival 177 in patients with metastatic GEP-NET receiving Lu-DOTATATE therapy? Hell. J. Nucl. Med. 2021;24:122–131. - PubMed
-
- Huizing D., Aalbersberg E.A., Versleijen M.W., Tesselaar M.E., Walraven I., Lahaye M.J., de Wit van der Veen B.J., Stokkel M.P. Early response assessment and prediction of overall survival after peptide receptor radionuclide therapy. Cancer Imaging. 2020;20:57. doi: 10.1186/s40644-020-00335-w. - DOI - PMC - PubMed
-
- Sjögreen Gleisner K., Chouin N., Gabina P.M., Cicone F., Gnesin S., Stokke C., Konijnenberg M., Cremonesi M., Verburg F.A., Bernhardt P. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor-and PSMA-targeting ligands. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:1778–1809. doi: 10.1007/s00259-022-05727-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

