A Nomogram and Risk Classification System Predicting the Prognosis of Patients with De Novo Metastatic Breast Cancer Undergoing Immediate Breast Reconstruction: A Surveillance, Epidemiology, and End Results Population-Based Study
- PMID: 38248093
- PMCID: PMC10814717
- DOI: 10.3390/curroncol31010008
A Nomogram and Risk Classification System Predicting the Prognosis of Patients with De Novo Metastatic Breast Cancer Undergoing Immediate Breast Reconstruction: A Surveillance, Epidemiology, and End Results Population-Based Study
Abstract
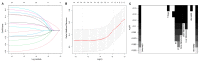
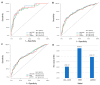
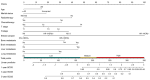
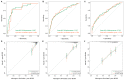
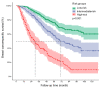
Background The lifespan of patients diagnosed with de novo metastatic breast cancer (dnMBC) has been prolonged. Nonetheless, there remains substantial debate regarding immediate breast reconstruction (IBR) for this particular subgroup of patients. The aim of this study was to construct a nomogram predicting the breast cancer-specific survival (BCSS) of dnMBC patients who underwent IBR. Methods A total of 682 patients initially diagnosed with metastatic breast cancer (MBC) between 2010 and 2018 in the Surveillance, Epidemiology, and End Results (SEER) database were included in this study. All patients were randomly allocated into training and validation groups at a ratio of 7:3. Univariate Cox hazard regression, least absolute shrinkage and selection operator (LASSO), and best subset regression (BSR) were used for initial variable selection, followed by a backward stepwise multivariate Cox regression to identify prognostic factors and construct a nomogram. Following the validation of the nomogram with concordance indexes (C-index), receiver operating characteristic (ROC) curves, calibration curves, and decision curve analyses (DCAs), risk stratifications were established. Results Age, marital status, T stage, N stage, breast subtype, bone metastasis, brain metastasis, liver metastasis, lung metastasis, radiotherapy, and chemotherapy were independent prognostic factors for BCSS. The C-indexes were 0.707 [95% confidence interval (CI), 0.666-0.748] in the training group and 0.702 (95% CI, 0.639-0.765) in the validation group. In the training group, the AUCs for BCSS were 0.857 (95% CI, 0.770-0.943), 0.747 (95% CI, 0.689-0.804), and 0.700 (95% CI, 0.643-0.757) at 1 year, 3 years, and 5 years, respectively, while in the validation group, the AUCs were 0.840 (95% CI, 0.733-0.947), 0.763 (95% CI, 0.677-0.849), and 0.709 (95% CI, 0.623-0.795) for the same time points. The calibration curves for BCSS probability prediction demonstrated excellent consistency. The DCA curves exhibited strong discrimination power and yielded substantial net benefits. Conclusions The nomogram, constructed based on prognostic risk factors, has the ability to provide personalized predictions for BCSS in dnMBC patients undergoing IBR and serve as a valuable reference for clinical decision making.
Keywords: SEER database; breast cancer-specific survival; de novo metastatic breast cancer; immediate breast reconstruction; nomogram.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Survival Nomogram for Patients With Locally Advanced Breast Cancer Undergoing Immediate Breast Reconstruction: A SEER Population-Based Study.Clin Breast Cancer. 2023 Jun;23(4):e219-e229. doi: 10.1016/j.clbc.2023.02.008. Epub 2023 Feb 21. Clin Breast Cancer. 2023. PMID: 36890005
-
A nomogram for predicting survival in patients with de novo metastatic breast cancer: a population-based study.BMC Cancer. 2020 Oct 12;20(1):982. doi: 10.1186/s12885-020-07449-1. BMC Cancer. 2020. PMID: 33046035 Free PMC article.
-
Establishment of Prognostic Nomogram for Male Breast Cancer Patients: A Surveillance, Epidemiology and End Results Database Analysis.Cancer Control. 2024 Jan-Dec;31:10732748241270628. doi: 10.1177/10732748241270628. Cancer Control. 2024. PMID: 39116271 Free PMC article.
-
Analysis of prognostic factors of metastatic endometrial cancer based on surveillance, epidemiology, and end results database.Front Surg. 2023 Jan 6;9:1001791. doi: 10.3389/fsurg.2022.1001791. eCollection 2022. Front Surg. 2023. PMID: 36684133 Free PMC article. Review.
-
Prognostic nomograms for young breast cancer: A retrospective study based on the SEER and METABRIC databases.Cancer Innov. 2024 Oct 25;3(6):e152. doi: 10.1002/cai2.152. eCollection 2024 Dec. Cancer Innov. 2024. PMID: 39464427 Free PMC article. Review.
Cited by
-
Individualized estimation of conditional survival for patients with spinal chordoma.Transl Cancer Res. 2025 Mar 30;14(3):1710-1724. doi: 10.21037/tcr-24-1912. Epub 2025 Mar 17. Transl Cancer Res. 2025. PMID: 40224976 Free PMC article.
-
Quality of Life After Locoregional Treatment in Women with De Novo Metastatic Breast Cancer: A Systematic Review and Meta-Analysis.Cancers (Basel). 2025 Feb 22;17(5):751. doi: 10.3390/cancers17050751. Cancers (Basel). 2025. PMID: 40075599 Free PMC article. Review.
-
Traditional Clinicopathological Biomarkers Still Determine Disease-Free and Overall Survival in Invasive Breast Cancer Patients: A Pilot Study.J Clin Med. 2024 Mar 30;13(7):2021. doi: 10.3390/jcm13072021. J Clin Med. 2024. PMID: 38610786 Free PMC article.
-
Breast Reconstruction in De Novo Metastatic Breast Cancer: A Systematic Review.Plast Reconstr Surg Glob Open. 2025 Jun 2;13(6):e6810. doi: 10.1097/GOX.0000000000006810. eCollection 2025 Jun. Plast Reconstr Surg Glob Open. 2025. PMID: 40469550 Free PMC article.
References
-
- Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J., et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

