Vanillic Acid Ameliorates Demyelination in a Cuprizone-Induced Multiple Sclerosis Rat Model: Possible Underlying Mechanisms
- PMID: 38248227
- PMCID: PMC10813517
- DOI: 10.3390/brainsci14010012
Vanillic Acid Ameliorates Demyelination in a Cuprizone-Induced Multiple Sclerosis Rat Model: Possible Underlying Mechanisms
Abstract
Objective: To investigate the effect of vanillic acid (VA) on a Cuprizone (Cup) demyelinating rat model and the mechanisms behind such effect.
Methods: Thirty adult male Sprague Dawley (SD) rats were randomly divided into three groups: control, Cuprizone, and VA groups. Cuprizone was administrated at a dose of 450 mg/kg per day orally via gastric gavage for 5 weeks. The nerve conduction velocity (NCV) was studied in an isolated sciatic nerve, and then the sciatic nerve was isolated for histopathological examination, electron microscope examination, immunohistochemical staining, and biochemical and PCR assay. The level of IL17 was detected using ELISA, while the antioxidant genes Nrf2, HO-1 expression at the level of mRNA, expression of the myelin basic protein (MBP), interferon-gamma factor (INF)-γ and tumor necrosis factor (TNF)-α, and apoptotic marker (caspase-3) were measured using immunohistochemistry in the sciatic nerve.
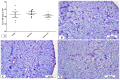
Results: There was a significant reduction in NCV in Cup compared to normal rats (p < 0.001), which was markedly improved in the VA group (p < 0.001). EM and histopathological examination revealed significant demyelination and deterioration of the sciatic nerve fibers with significant improvement in the VA group. The level of IL17 as well as the expression of INF-γ and caspase-3 were significantly increased with a significant reduction in the expression of MBP, Nrf2, and HO-1 in the sciatic nerve (p < 0.01), and VA treatment significantly improved the studied parameters (p < 0.01).
Conclusion: The current study demonstrated a neuroprotective effect for VA against the Cup-induced demyelinating rat model. This effect might be precipitated by the inhibition of inflammation, oxidative stress, and apoptosis.
Keywords: Nrf2; cuprizone; demyelinating; neuroinflammation; vanillic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Pirfenidone mitigates demyelination and electrophysiological alterations in multiple sclerosis: Targeting NF-κB, sirt1, and neurotrophic genes.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):4019-4036. doi: 10.1007/s00210-024-03496-8. Epub 2024 Oct 15. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39404841 Free PMC article.
-
New Insight into the Possible Roles of L-Carnitine in a Rat Model of Multiple Sclerosis.Brain Sci. 2023 Dec 25;14(1):23. doi: 10.3390/brainsci14010023. Brain Sci. 2023. PMID: 38248238 Free PMC article.
-
Neuroprotective Effects of Telmisartan and Nifedipine Against Cuprizone-Induced Demyelination and Behavioral Dysfunction in Mice: Roles of NF-κB and Nrf2.Inflammation. 2021 Aug;44(4):1629-1642. doi: 10.1007/s10753-021-01447-6. Epub 2021 Mar 11. Inflammation. 2021. PMID: 33709265
-
Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axis and restriction of mature oligodendrocytes apoptosis.Pharm Biol. 2017 Dec;55(1):1679-1687. doi: 10.1080/13880209.2017.1319867. Pharm Biol. 2017. Retraction in: Pharm Biol. 2024 Dec;62(1):562. doi: 10.1080/13880209.2024.2374677. PMID: 28447514 Free PMC article. Retracted.
-
Coenzyme Q10 enhances remyelination and regulate inflammation effects of cuprizone in corpus callosum of chronic model of multiple sclerosis.J Mol Histol. 2021 Feb;52(1):125-134. doi: 10.1007/s10735-020-09929-x. Epub 2020 Nov 27. J Mol Histol. 2021. PMID: 33245472
Cited by
-
Berberine-loaded iron oxide nanoparticles alleviate cuprizone-induced astrocytic reactivity in a rat model of multiple sclerosis.Biometals. 2025 Feb;38(1):203-229. doi: 10.1007/s10534-024-00648-4. Epub 2024 Nov 14. Biometals. 2025. PMID: 39543075 Free PMC article.
-
Neurotherapeutic impact of vanillic acid and ibudilast on the cuprizone model of multiple sclerosis.Front Mol Neurosci. 2025 Jan 10;17:1503396. doi: 10.3389/fnmol.2024.1503396. eCollection 2024. Front Mol Neurosci. 2025. PMID: 39866908 Free PMC article.
-
The treatment of primary CoQ deficiency requires the targeting of multiple pathogenic mechanisms.Commun Med (Lond). 2025 Jul 10;5(1):286. doi: 10.1038/s43856-025-01000-8. Commun Med (Lond). 2025. PMID: 40640372 Free PMC article.
-
Antioxidant Therapies in the Treatment of Multiple Sclerosis.Biomolecules. 2024 Oct 8;14(10):1266. doi: 10.3390/biom14101266. Biomolecules. 2024. PMID: 39456199 Free PMC article. Review.
-
Pirfenidone mitigates demyelination and electrophysiological alterations in multiple sclerosis: Targeting NF-κB, sirt1, and neurotrophic genes.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):4019-4036. doi: 10.1007/s00210-024-03496-8. Epub 2024 Oct 15. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39404841 Free PMC article.
References
-
- Abdel-maged A.E., Gad A.M., Rashed I.A., Azab S.S., Mohamed E.A., Awad A.S. Repurposing of secukinumab as neuroprotective in cuprizone-induced multiple sclerosis experimental model via inhibition of oxidative, inflammatory, and neurodegenerative signaling. Mol. Neurobiol. 2020;57:3291–3306. doi: 10.1007/s12035-020-01972-9. - DOI - PubMed
-
- Begum Z., Vijayalakshmi S., Raghunath G. Cuprizone Induced Demyelination in Male Wistar Rats with Timeline Changes Admistered through Oral Gavage. Ann. Rom. Soc. Cell Biol. 2021;25:12643–12653.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

