Exploring Vitreous Haze as a Potential Biomarker for Accelerated Glymphatic Outflow and Neurodegeneration in Multiple Sclerosis: A Cross-Sectional Study
- PMID: 38248251
- PMCID: PMC10813039
- DOI: 10.3390/brainsci14010036
Exploring Vitreous Haze as a Potential Biomarker for Accelerated Glymphatic Outflow and Neurodegeneration in Multiple Sclerosis: A Cross-Sectional Study
Abstract
Background: The glymphatic system removes neurodegenerative debris. The ocular glymphatic outflow is from the eye to the proximal optic nerve. In multiple sclerosis (MS), atrophy of the optic nerve increases the glymphatic outflow space. Here, we tested whether vitreous haze (VH) can provide novel insights into the relationship between neurodegeneration and the ocular glymphatic system in MS.
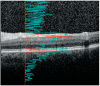
Methods: This cross-sectional study comprised 315 persons with MS and 87 healthy controls (HCs). VH was quantified from optical coherence tomography (OCT) volume scans. Neurodegeneration was determined on three-dimensional T1 (3DT1) MRI, lesion detection on fluid-attenuated inversion (FLAIR), and layer thickness on OCT. Generalized estimating equations, corrected for age, were used to analyze associations between VH and metrics for neurodegeneration, demographics, and clinical scales. Group differences were determined between mild, moderate, and severe disability.
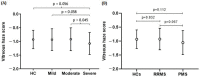
Results: On the group level, VH scores were comparable between MS and control (p = 0.629). In MS, VH scores declined with disease duration (β = -0.009, p = 0.004) and age (β = -0.007, p = 0.001). There was no relation between VH scores and higher age in HCs. In MS patients, VH was related to normalized gray (NGMV, β = 0.001, p = 0.011) and white matter volume (NWMV, β = 0.001, p = 0.003), macular ganglion cell-inner plexiform layer thickness (mGCIPL, β = 0.006, p < 0.001), and peripapillary retinal nerve fiber layer thickness (pRNFL, β = 0.004, p = 0.008). VH was significantly lower in severe compared to mild disability (mean difference -28.86%, p = 0.058).
Conclusions: There is a correlation between VH on OCT and disease duration, more severe disability and lower brain volumes in MS. Biologically, these relationships suggest accelerated glymphatic clearance with disease-related atrophy.
Keywords: glymphatic system; multiple sclerosis; neurodegeneration; optical coherence tomography; vitreous haze.
Conflict of interest statement
All authors declare that they have no relevant potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Objective quantification of vitreous haze on optical coherence tomography scans: no evidence for relationship between uveitis and inflammation in multiple sclerosis.Eur J Neurol. 2020 Jan;27(1):144-e3. doi: 10.1111/ene.14048. Epub 2019 Aug 18. Eur J Neurol. 2020. PMID: 31342606 Free PMC article.
-
Optical coherence tomography in multiple sclerosis: A Tunisian tertiary center study.J Fr Ophtalmol. 2025 Feb;48(2):104371. doi: 10.1016/j.jfo.2024.104371. Epub 2024 Dec 10. J Fr Ophtalmol. 2025. PMID: 39662309
-
Anatomy-driven segmentation of parafoveal optical coherence tomography (OCT) measures may improve associations with clinical outcomes in multiple sclerosis.J Neurol. 2025 Jan 15;272(2):141. doi: 10.1007/s00415-024-12866-4. J Neurol. 2025. PMID: 39812862
-
Can Optical Coherence Tomography Be Used to Guide Treatment Decisions in Adult or Pediatric Multiple Sclerosis?Curr Treat Options Neurol. 2018 Mar 21;20(4):9. doi: 10.1007/s11940-018-0493-6. Curr Treat Options Neurol. 2018. PMID: 29564662 Review.
-
Optical coherence tomography as a prognostic tool for disability progression in MS: a systematic review.J Neurol. 2023 Feb;270(2):1178-1186. doi: 10.1007/s00415-022-11474-4. Epub 2022 Nov 13. J Neurol. 2023. PMID: 36372866
References
-
- Criste G., Trapp B., Dutta R. Chapter 5—Axonal loss in multiple sclerosis: Causes and mechanisms. In: Goodin D.S., editor. Handbook of Clinical Neurology. Volume 122. Elsevier; Amsterdam, The Netherlands: 2014. pp. 101–113. - PubMed
-
- Walton C., King R., Rechtman L., Kaye W., Leray E., Marrie R.A., Robertson N., La Rocca N., Uitdehaag B., van der Mei I., et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020;26:1816–1821. doi: 10.1177/1352458520970841. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

