A Novel Acetone Sensor for Body Fluids
- PMID: 38248381
- PMCID: PMC10813317
- DOI: 10.3390/bios14010004
A Novel Acetone Sensor for Body Fluids
Abstract
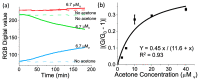
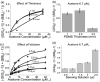
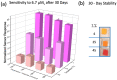
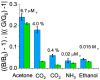
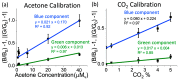
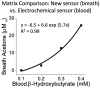
Ketones are well-known biomarkers of fat oxidation produced in the liver as a result of lipolysis. These biomarkers include acetoacetic acid and β-hydroxybutyric acid in the blood/urine and acetone in our breath and skin. Monitoring ketone production in the body is essential for people who use caloric intake deficit to reduce body weight or use ketogenic diets for wellness or therapeutic treatments. Current methods to monitor ketones include urine dipsticks, capillary blood monitors, and breath analyzers. However, these existing methods have certain disadvantages that preclude them from being used more widely. In this work, we introduce a novel acetone sensor device that can detect acetone levels in breath and overcome the drawbacks of existing sensing approaches. The critical element of the device is a robust sensor with the capability to measure acetone using a complementary metal oxide semiconductor (CMOS) chip and convenient data analysis from a red, green, and blue deconvolution imaging approach. The acetone sensor device demonstrated sensitivity of detection in the micromolar-concentration range, selectivity for detection of acetone in breath, and a lifetime stability of at least one month. The sensor device utility was probed with real tests on breath samples using an established blood ketone reference method.
Keywords: breath sensor; digital medicine; fat burning; fat oxidation; ketones; metabolic rate; point of care; wearable sensor.
Conflict of interest statement
Arizona State University owns intellectual property related to the measurement technology: U.S. Patent Application No. 20210048206. T.F. Health Corporation has exclusive license of this patent. Erica Forzani is co-founder of T.F. Health Corporation. Xiaojun Xian is VP of Production of T.F. Health Corporation.
Figures


References
-
- Tsow F., Xian X.J., Bridgeman D., Forzani E.S., Tao N.J. Self-Contained Wearable Metabolic Analyzer. US20210378546A1. 2021 December 9;
-
- Forzani E.S., Tao N.J. Metabolic Analyzer. US20130150746. 2013 June 13;
-
- Tsow F., Xian X., Forzani E., Tao N.J. An Improved Portable Metabolic Analyzer System. US10250369795A1. 2015 December 24;
-
- Tao N.J., Forzani E.S. Metabolic Analyzer. U.S. Patent 10,143,401. 2018 December 4;
-
- Tsow F., Xian X.J., Forzani E.S., Tao N.J. Portable Metabolic Analyzer. U.S. Patent 10,078,074. 2018 September 18;
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

