Gram-Positive Bacterial Membrane-Based Biosensor for Multimodal Investigation of Membrane-Antibiotic Interactions
- PMID: 38248423
- PMCID: PMC10813107
- DOI: 10.3390/bios14010045
Gram-Positive Bacterial Membrane-Based Biosensor for Multimodal Investigation of Membrane-Antibiotic Interactions
Abstract
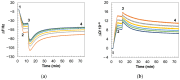
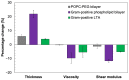
As membrane-mediated antibiotic resistance continues to evolve in Gram-positive bacteria, the development of new approaches to elucidate the membrane properties involved in antibiotic resistance has become critical. Membrane vesicles (MVs) secreted by the cytoplasmic membrane of Gram-positive bacteria contain native components, preserving lipid and protein diversity, nucleic acids, and sometimes virulence factors. Thus, MV-derived membrane platforms present a great model for Gram-positive bacterial membranes. In this work, we report the development of a planar bacterial cytoplasmic membrane-based biosensor using MVs isolated from the Bacillus subtilis WT strain that can be coated on multiple surface types such as glass, quartz crystals, and polymeric electrodes, fostering the multimodal assessment of drug-membrane interactions. Retention of native membrane components such as lipoteichoic acids, lipids, and proteins is verified. This biosensor replicates known interaction patterns of the antimicrobial compound, daptomycin, with the Gram-positive bacterial membrane, establishing the applicability of this platform for carrying out biophysical characterization of the interactions of membrane-acting antibiotic compounds with the bacterial cytoplasmic membrane. We report changes in membrane viscoelasticity and permeability that correspond to partial membrane disruption when calcium ions are present with daptomycin but not when these ions are chelated. This biomembrane biosensing platform enables an assessment of membrane biophysical characteristics during exposure to antibiotic drug candidates to aid in identifying compounds that target membrane disruption as a mechanism of action.
Keywords: Gram-positive bacteria; antibiotic sensing; daptomycin; membrane vesicles; microelectrode array; organic electronic; permeability; supported lipid bilayer.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Figures






Similar articles
-
Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin (LY146032).Antimicrob Agents Chemother. 1990 Jun;34(6):1220-6. doi: 10.1128/AAC.34.6.1220. Antimicrob Agents Chemother. 1990. PMID: 2168145 Free PMC article.
-
Lipid membrane-binding properties of daptomycin using surface plasmon resonance.Anal Sci. 2013;29(3):297-301. doi: 10.2116/analsci.29.297. Anal Sci. 2013. PMID: 23474718
-
Oligomerization of daptomycin on membranes.Biochim Biophys Acta. 2011 Apr;1808(4):1154-60. doi: 10.1016/j.bbamem.2011.01.001. Epub 2011 Jan 9. Biochim Biophys Acta. 2011. PMID: 21223947
-
The role of bacterial membrane vesicles in antibiotic resistance.Ann N Y Acad Sci. 2023 Jan;1519(1):63-73. doi: 10.1111/nyas.14932. Epub 2022 Nov 22. Ann N Y Acad Sci. 2023. PMID: 36415037 Review.
-
Mechanisms of drug resistance: daptomycin resistance.Ann N Y Acad Sci. 2015 Sep;1354:32-53. doi: 10.1111/nyas.12948. Epub 2015 Oct 23. Ann N Y Acad Sci. 2015. PMID: 26495887 Free PMC article. Review.
Cited by
-
Engineering Planar Gram-Negative Outer Membrane Mimics Using Bacterial Outer Membrane Vesicles.Langmuir. 2024 Nov 5;40(44):23289-23300. doi: 10.1021/acs.langmuir.4c02632. Epub 2024 Oct 25. Langmuir. 2024. PMID: 39453730 Free PMC article.
-
Engineering Planar Gram-Negative Outer Membrane Mimics Using Bacterial Outer Membrane Vesicles.bioRxiv [Preprint]. 2024 Aug 20:2023.12.11.570829. doi: 10.1101/2023.12.11.570829. bioRxiv. 2024. Update in: Langmuir. 2024 Nov 5;40(44):23289-23300. doi: 10.1021/acs.langmuir.4c02632. PMID: 39229024 Free PMC article. Updated. Preprint.
References
-
- Kamar R., Réjasse A., Jéhanno I., Attieh Z., Courtin P., Chapot-Chartier M.-P., Nielsen-Leroux C., Lereclus D., el Chamy L., Kallassy M., et al. DltX of Bacillus Thuringiensis Is Essential for D-Alanylation of Teichoic Acids and Resistance to Antimicrobial Response in Insects. Front. Microbiol. 2017;8:01437. doi: 10.3389/fmicb.2017.01437. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

