Seasonal Changes in the Soil Microbial Community Structure in Urban Forests
- PMID: 38248462
- PMCID: PMC10813005
- DOI: 10.3390/biology13010031
Seasonal Changes in the Soil Microbial Community Structure in Urban Forests
Abstract
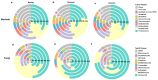
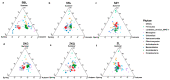
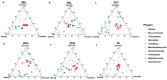
Urban forests play a crucial role in the overall health and stability of urban ecosystems. Soil microorganisms are vital to the functioning of urban forest ecosystems as they facilitate material cycling and contribute to environmental stability. This study utilized high-throughput sequencing technology to examine the structural characteristics of bacterial and fungal communities in the bulk soil of six different forest stands: Phyllostachys pubescens (ZL), Metasequoia glyptostroboides (SSL), Cornus officinalis (SZY), mixed broad-leaved shrub forest (ZKG), mixed pine and cypress forest (SBL), and mixed broad-leaved tree forest (ZKQ). Soil samples were collected from each forest stand, including the corners, center, and edges of each plot, and a combined sample was created from the first five samples. The results revealed that among the bacterial communities, ZKG exhibited the highest alpha diversity in spring, while ZL demonstrated the highest alpha diversity in both summer and autumn. Proteobacteria was the most abundant bacterial phylum in all six forest stand soils. The dominant fungal phylum across the six forest stands was identified as Ascomycota. Notably, the microbial community diversity of SBL bulk soil exhibited significant seasonal changes. Although ZL exhibited lower bacterial community diversity in spring, its fungal community diversity was the highest. The bulk soil microbial diversity of ZL and SSL surpassed that of the other forest stands, suggesting their importance in maintaining the stability of the urban forest ecosystem in the Zhuyu Bay Scenic Area. Furthermore, the diversity of the bulk soil microbial communities was higher in all six stands during spring compared to summer and autumn. Overall, this study provides valuable insights into the seasonal variations of bulk soil microbial communities in urban forests and identifies dominant tree species, offering guidance for tree species' selection and preservation in urban forest management.
Keywords: community diversity; seasonal changes; soil microbial; tree species; urban forest.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Microbiota associated with urban forests.PeerJ. 2024 Feb 29;12:e16987. doi: 10.7717/peerj.16987. eCollection 2024. PeerJ. 2024. PMID: 38444615 Free PMC article.
-
Different Urban Forest Tree Species Affect the Assembly of the Soil Bacterial and Fungal Community.Microb Ecol. 2022 Feb;83(2):447-458. doi: 10.1007/s00248-021-01754-3. Epub 2021 May 24. Microb Ecol. 2022. PMID: 34031701
-
Soil bacterial community structure of mixed bamboo and broad-leaved forest based on tree crown width ratio.Sci Rep. 2020 Apr 16;10(1):6522. doi: 10.1038/s41598-020-63547-x. Sci Rep. 2020. PMID: 32300174 Free PMC article.
-
Soil fungal community and co-occurrence network patterns at different successional stages of black locust coppice stands.Front Microbiol. 2025 Mar 18;16:1528028. doi: 10.3389/fmicb.2025.1528028. eCollection 2025. Front Microbiol. 2025. PMID: 40170928 Free PMC article.
-
A comparison of microbial composition under three tree ecosystems using the stochastic process and network complexity approaches.Front Microbiol. 2022 Oct 10;13:1018077. doi: 10.3389/fmicb.2022.1018077. eCollection 2022. Front Microbiol. 2022. PMID: 36299726 Free PMC article.
Cited by
-
Subtle changes in topsoil microbial communities of drained forested peatlands after prolonged drought.Environ Microbiol Rep. 2024 Dec;16(6):e70041. doi: 10.1111/1758-2229.70041. Environ Microbiol Rep. 2024. PMID: 39512007 Free PMC article.
-
Synergistic Effects of Paenibacillus polymyxa NBmelon-1 Inoculation and Grafting Restructure of Rhizosphere Microbiome and Enhanced Disease Resistance in Melon Self-Rootstocks.Microorganisms. 2025 May 22;13(6):1172. doi: 10.3390/microorganisms13061172. Microorganisms. 2025. PMID: 40572060 Free PMC article.
References
-
- Klimek B., Chodak M., Jaźwa M., Niklińska M. Functional diversity of soil microbial communities in boreal and temperate Scots pine forests. Eur. J. For. Res. 2016;135:731–742. doi: 10.1007/s10342-016-0968-5. - DOI
-
- Li J., Li Z., Wang F., Zou B., Chen Y., Zhao J., Mo Q., Li Y., Li X., Xia H. Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol. Fertil. Soils. 2015;51:207–215. doi: 10.1007/s00374-014-0964-1. - DOI
-
- Wu H., Zeng G., Liang J., Guo S., Dai J., Lu L., Wei Z., Xu P., Li F., Yuan Y., et al. Effect of early dry season induced by the Three Gorges Dam on the soil microbial biomass and bacterial community structure in the Dongting Lake wetland. Ecol. Indic. 2015;53:129–136. doi: 10.1016/j.ecolind.2015.01.041. - DOI
LinkOut - more resources
Full Text Sources

