Lipid Metabolism in Metabolic-Associated Steatotic Liver Disease (MASLD)
- PMID: 38248815
- PMCID: PMC10818604
- DOI: 10.3390/metabo14010012
Lipid Metabolism in Metabolic-Associated Steatotic Liver Disease (MASLD)
Abstract
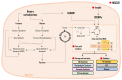
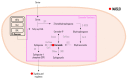
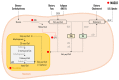
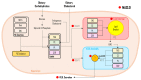
Metabolic-associated steatotic liver disease (MASLD) is a cluster of pathological conditions primarily developed due to the accumulation of ectopic fat in the hepatocytes. During the severe form of the disease, i.e., metabolic-associated steatohepatitis (MASH), accumulated lipids promote lipotoxicity, resulting in cellular inflammation, oxidative stress, and hepatocellular ballooning. If left untreated, the advanced form of the disease progresses to fibrosis of the tissue, resulting in irreversible hepatic cirrhosis or the development of hepatocellular carcinoma. Although numerous mechanisms have been identified as significant contributors to the development and advancement of MASLD, altered lipid metabolism continues to stand out as a major factor contributing to the disease. This paper briefly discusses the dysregulation in lipid metabolism during various stages of MASLD.
Keywords: DNL; MASH; MASLD; cholesterol; fatty acid oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Hepatic angiotensin-converting enzyme 2 expression in metabolic dysfunction-associated steatotic liver disease and in patients with fatal COVID-19.World J Gastroenterol. 2024 Aug 21;30(31):3705-3716. doi: 10.3748/wjg.v30.i31.3705. World J Gastroenterol. 2024. PMID: 39192998 Free PMC article.
-
NAFLD (MASLD)/NASH (MASH): Does It Bother to Label at All? A Comprehensive Narrative Review.Int J Mol Sci. 2024 Aug 2;25(15):8462. doi: 10.3390/ijms25158462. Int J Mol Sci. 2024. PMID: 39126031 Free PMC article. Review.
-
Therapeutic implications for sphingolipid metabolism in metabolic dysfunction-associated steatohepatitis.Front Endocrinol (Lausanne). 2024 Jun 19;15:1400961. doi: 10.3389/fendo.2024.1400961. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38962680 Free PMC article. Review.
-
Lipotoxicity-driven metabolic dysfunction-associated steatotic liver disease (MASLD).Atherosclerosis. 2025 Jan;400:119053. doi: 10.1016/j.atherosclerosis.2024.119053. Epub 2024 Nov 14. Atherosclerosis. 2025. PMID: 39581063 Review.
-
Role of genetic variants and DNA methylation of lipid metabolism-related genes in metabolic dysfunction-associated steatotic liver disease.Front Physiol. 2025 Mar 17;16:1562848. doi: 10.3389/fphys.2025.1562848. eCollection 2025. Front Physiol. 2025. PMID: 40166716 Free PMC article. Review.
Cited by
-
Helicobacter pylori infection is linked to metabolic dysfunction and associated steatotic liver disease: A large cross-sectional study.World J Gastroenterol. 2025 Apr 7;31(13):102563. doi: 10.3748/wjg.v31.i13.102563. World J Gastroenterol. 2025. PMID: 40248064 Free PMC article.
-
Bridging systemic metabolic dysfunction and Alzheimer's disease: the liver interface.Mol Neurodegener. 2025 May 28;20(1):61. doi: 10.1186/s13024-025-00849-6. Mol Neurodegener. 2025. PMID: 40437610 Free PMC article. Review.
-
Thyroid hormone and the Liver.Hepatol Commun. 2024 Dec 20;9(1):e0596. doi: 10.1097/HC9.0000000000000596. eCollection 2025 Jan 1. Hepatol Commun. 2024. PMID: 39699315 Free PMC article. Review.
-
Alcohol Consumption and Liver Metabolism in the Era of MASLD: Integrating Nutritional and Pathophysiological Insights.Nutrients. 2025 Jul 5;17(13):2229. doi: 10.3390/nu17132229. Nutrients. 2025. PMID: 40647333 Free PMC article. Review.
-
Aerobic capacity and exercise mediate protection against hepatic steatosis via enhanced bile acid metabolism.bioRxiv [Preprint]. 2024 Oct 24:2024.10.21.619494. doi: 10.1101/2024.10.21.619494. bioRxiv. 2024. Update in: Function (Oxf). 2025 May 19;6(3):zqaf019. doi: 10.1093/function/zqaf019. PMID: 39484384 Free PMC article. Updated. Preprint.
References
-
- Rinella M.E., Lazarus J.V., Ratziu V., Francque S.M., Sanyal A.J., Kanwal F., Romero D., Abdelmalek M.F., Anstee Q.M., Arab J.P., et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966–1986. doi: 10.1097/HEP.0000000000000520. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

