Bayesian 13C-Metabolic Flux Analysis of Parallel Tracer Experiments in Granulocytes: A Directional Shift within the Non-Oxidative Pentose Phosphate Pathway Supports Phagocytosis
- PMID: 38248827
- PMCID: PMC10820746
- DOI: 10.3390/metabo14010024
Bayesian 13C-Metabolic Flux Analysis of Parallel Tracer Experiments in Granulocytes: A Directional Shift within the Non-Oxidative Pentose Phosphate Pathway Supports Phagocytosis
Abstract
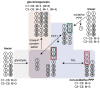
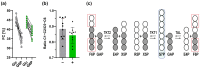
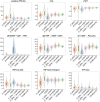
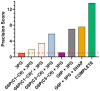
The pentose phosphate pathway (PPP) plays a key role in the cellular regulation of immune function; however, little is known about the interplay of metabolic adjustments in granulocytes, especially regarding the non-oxidative PPP. For the determination of metabolic mechanisms within glucose metabolism, we propose a novel set of measures for 13C-metabolic flux analysis based on ex vivo parallel tracer experiments ([1,2-13C]glucose, [U-13C]glucose, [4,5,6-13C]glucose) and gas chromatography-mass spectrometry labeling measurements of intracellular metabolites, such as sugar phosphates and their fragments. A detailed constraint analysis showed that the permission range for net and irreversible fluxes was limited to a three-dimensional space. The overall workflow, including its Bayesian flux estimation, resulted in precise flux distributions and pairwise confidence intervals, some of which could be represented as a line due to the strength of their correlation. The principal component analysis that was enabled by these behaviors comprised three components that explained 99.6% of the data variance. It showed that phagocytic stimulation reversed the direction of non-oxidative PPP net fluxes from ribose-5-phosphate biosynthesis toward glycolytic pathways. This process was closely associated with the up-regulation of the oxidative PPP to promote the oxidative burst.
Keywords: Bayesian modeling; gas chromatography–mass spectrometry; glucose metabolism; immunometabolism; isotopic tracer; mass isotopomer distribution analysis; metabolic model validation; phagocytose; principal component analysis; sugar phosphates.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








Similar articles
-
Ex Vivo 13C-Metabolic Flux Analysis of Porcine Circulating Immune Cells Reveals Cell Type-Specific Metabolic Patterns and Sex Differences in the Pentose Phosphate Pathway.Biomolecules. 2024 Jan 12;14(1):98. doi: 10.3390/biom14010098. Biomolecules. 2024. PMID: 38254698 Free PMC article.
-
Evidence for transketolase-like TKTL1 flux in CHO cells based on parallel labeling experiments and (13)C-metabolic flux analysis.Metab Eng. 2016 Sep;37:72-78. doi: 10.1016/j.ymben.2016.05.005. Epub 2016 May 10. Metab Eng. 2016. PMID: 27174718 Free PMC article.
-
Updates to a 13C metabolic flux analysis model for evaluating energy metabolism in cultured cerebellar granule neurons from neonatal rats.Neurochem Int. 2017 Oct;109:54-67. doi: 10.1016/j.neuint.2017.03.020. Epub 2017 Apr 13. Neurochem Int. 2017. PMID: 28412312 Free PMC article.
-
Isotopic Tracer for Absolute Quantification of Metabolites of the Pentose Phosphate Pathway in Bacteria.Metabolites. 2022 Nov 9;12(11):1085. doi: 10.3390/metabo12111085. Metabolites. 2022. PMID: 36355168 Free PMC article. Review.
-
Pentose Phosphate Pathway Reactions in Photosynthesizing Cells.Cells. 2021 Jun 18;10(6):1547. doi: 10.3390/cells10061547. Cells. 2021. PMID: 34207480 Free PMC article. Review.
Cited by
-
Ex Vivo 13C-Metabolic Flux Analysis of Porcine Circulating Immune Cells Reveals Cell Type-Specific Metabolic Patterns and Sex Differences in the Pentose Phosphate Pathway.Biomolecules. 2024 Jan 12;14(1):98. doi: 10.3390/biom14010098. Biomolecules. 2024. PMID: 38254698 Free PMC article.
-
13C-Metabolic flux analysis detected a hyperoxemia-induced reduction of tricarboxylic acid cycle metabolism in granulocytes during two models of porcine acute subdural hematoma and hemorrhagic shock.Front Immunol. 2024 Jan 9;14:1319986. doi: 10.3389/fimmu.2023.1319986. eCollection 2023. Front Immunol. 2024. PMID: 38332911 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

