Altered Sphingolipid Hydrolase Activities and Alpha-Synuclein Level in Late-Onset Schizophrenia
- PMID: 38248833
- PMCID: PMC10819534
- DOI: 10.3390/metabo14010030
Altered Sphingolipid Hydrolase Activities and Alpha-Synuclein Level in Late-Onset Schizophrenia
Abstract
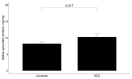
Recent data described that patients with lysosomal storage disorders (LSDs) may have clinical schizophrenia (SCZ) features. Disruption of lipid metabolism in SCZ pathogenesis was found. Clinical features of schizophrenia (SCZ) have been demonstrated in patients with several lysosomal storage disorders (LSDs). Taking into account the critical role of lysosomal function for neuronal cells' lysosomal dysfunction could be proposed in SCZ pathogenesis. The current study analyzed lysosomal enzyme activities and the alpha-synuclein level in the blood of patients with late-onset SCZ. In total, 52 SCZ patients with late-onset SCZ, 180 sporadic Parkinson's disease (sPD) patients, and 176 controls were recruited. The enzymatic activity of enzymes associated with mucopolysaccharidosis (alpha-L-Iduronidase (IDUA)), glycogenosis (acid alpha-glucosidase (GAA)) and sphingolipidosis (galactosylceramidase (GALC), glucocerebrosidase (GCase), alpha-galactosidase (GLA), acid sphingomyelinase (ASMase)) and concentration of lysosphingolipids (hexosylsphingosine (HexSph), globotriaosylsphingosine (LysoGb3), and lysosphingomyelin (LysoSM)) were measured using LC-MS/MS. The alpha-synuclein level was estimated in magnetically separated CD45+ blood cells using the enzyme-linked immunosorbent assay (ELISA). Additionally, NGS analysis of 11 LSDs genes was conducted in 21 early-onset SCZ patients and 23 controls using the gene panel PGRNseq-NDD. Decreased ASMase, increased GLA activities, and increased HexSpn, LysoGb3, and LysoSM concentrations along with an accumulation of the alpha-synuclein level were observed in late-onset SCZ patients in comparison to the controls (p < 0.05). Four rare deleterious variants among LSDs genes causing mucopolysaccharidosis type I (IDUA (rs532731688, rs74385837) and type III (HGSNAT (rs766835582)) and sphingolipidosis (metachromatic leukodystrophy (ARSA (rs201251634)) were identified in five patients from the group of early-onset SCZ patients but not in the controls. Our findings supported the role of sphingolipid metabolism in SCZ pathogenesis. Aberrant enzyme activities and compounds of sphingolipids associated with ceramide metabolism may lead to accumulation of alpha-synuclein and may be critical in SCZ pathogenesis.
Keywords: Parkinson’s disease; alpha-synuclein; hydrolase activity; lysosomal storage disorder genes; schizophrenia; sphingolipids.
Conflict of interest statement
Yury A. Barbitoff and Oleg S. Glotov work both in the institute and in the company. Yury Barbitoff and Oleg Glotov are employees of Cerbalab Ltd (Saint Petersburg, Russia, https://cerbalab.ru). The paper reflects the views of the scientists, and not the company.
Figures
References
-
- Howrigan D.P., Rose S.A., Samocha K.E., Fromer M., Cerrato F., Chen W.J., Churchhouse C., Chambert K., Chandler S.D., Daly M.J., et al. Exome Sequencing in Schizophrenia-Affected Parent–Offspring Trios Reveals Risk Conferred by Protein-Coding de Novo Mutations. Nat. Neurosci. 2020;23:185–193. doi: 10.1038/s41593-019-0564-3. - DOI - PMC - PubMed
-
- Oh J., Shen G., Nan G., Kim J.M., Jung K.Y., Jeon B. Comorbid Schizophrenia and Parkinson’s Disease: A Case Series and Brief Review. Neurol. Asia. 2017;22:139–142.
-
- Pardiñas A.F., Holmans P., Pocklington A.J., Escott-Price V., Ripke S., Carrera N., Legge S.E., Bishop S., Cameron D., Hamshere M.L., et al. Common Schizophrenia Alleles Are Enriched in Mutation-Intolerant Genes and in Regions under Strong Background Selection. Nat. Genet. 2018;50:381–389. doi: 10.1038/s41588-018-0059-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous