Endpoints in NASH Clinical Trials: Are We Blind in One Eye?
- PMID: 38248843
- PMCID: PMC10820221
- DOI: 10.3390/metabo14010040
Endpoints in NASH Clinical Trials: Are We Blind in One Eye?
Abstract
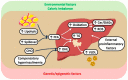
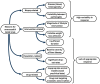
This narrative review aims to illustrate the notion that nonalcoholic steatohepatitis (NASH), recently renamed metabolic dysfunction-associated steatohepatitis (MASH), is a systemic metabolic disorder featuring both adverse hepatic and extrahepatic outcomes. In recent years, several NASH trials have failed to identify effective pharmacological treatments and, therefore, lifestyle changes are the cornerstone of therapy for NASH. with this context, we analyze the epidemiological burden of NASH and the possible pathogenetic factors involved. These include genetic factors, insulin resistance, lipotoxicity, immuno-thrombosis, oxidative stress, reprogramming of hepatic metabolism, and hypoxia, all of which eventually culminate in low-grade chronic inflammation and increased risk of fibrosis progression. The possible explanations underlying the failure of NASH trials are also accurately examined. We conclude that the high heterogeneity of NASH, resulting from variable genetic backgrounds, exposure, and responses to different metabolic stresses, susceptibility to hepatocyte lipotoxicity, and differences in repair-response, calls for personalized medicine approaches involving research on noninvasive biomarkers. Future NASH trials should aim at achieving a complete assessment of systemic determinants, modifiers, and correlates of NASH, thus adopting a more holistic and unbiased approach, notably including cardiovascular-kidney-metabolic outcomes, without restricting therapeutic perspectives to histological surrogates of liver-related outcomes alone.
Keywords: biomarkers; cardiovascular risk; liver biopsy; metabolic dysfunction; natural history.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Emerging and Established Therapeutic Approaches for Nonalcoholic Fatty Liver Disease.Clin Ther. 2021 Sep;43(9):1476-1504. doi: 10.1016/j.clinthera.2021.07.013. Epub 2021 Aug 24. Clin Ther. 2021. PMID: 34446271 Review.
-
Nonalcoholic steatohepatitis and mechanisms by which it is ameliorated by activation of the CNC-bZIP transcription factor Nrf2.Free Radic Biol Med. 2022 Aug 1;188:221-261. doi: 10.1016/j.freeradbiomed.2022.06.226. Epub 2022 Jun 18. Free Radic Biol Med. 2022. PMID: 35728768 Review.
-
FGF21 protects against hepatic lipotoxicity and macrophage activation to attenuate fibrogenesis in nonalcoholic steatohepatitis.Elife. 2023 Jan 17;12:e83075. doi: 10.7554/eLife.83075. Elife. 2023. PMID: 36648330 Free PMC article.
-
Etiopathogenesis of nonalcoholic steatohepatitis.Semin Liver Dis. 2001;21(1):27-41. doi: 10.1055/s-2001-12927. Semin Liver Dis. 2001. PMID: 11296694 Review.
-
Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease.Adv Exp Med Biol. 2018;1061:19-44. doi: 10.1007/978-981-10-8684-7_3. Adv Exp Med Biol. 2018. PMID: 29956204 Review.
Cited by
-
Red cell distribution width/platelet ratio predicts decompensation of metabolic dysfunction-associated steatotic liver disease-related compensated advanced chronic liver disease.World J Gastroenterol. 2025 Jan 21;31(3):100393. doi: 10.3748/wjg.v31.i3.100393. World J Gastroenterol. 2025. PMID: 39839903 Free PMC article.
-
Molecular Pharmacology of Vitamin C and Relevance to Health and Obesity-A Narrative Review.Int J Mol Sci. 2024 Jul 9;25(14):7523. doi: 10.3390/ijms25147523. Int J Mol Sci. 2024. PMID: 39062764 Free PMC article. Review.
-
Does an Aspirin a Day Take the MASLD Away?Adv Ther. 2024 Jul;41(7):2559-2575. doi: 10.1007/s12325-024-02885-y. Epub 2024 May 15. Adv Ther. 2024. PMID: 38748333 Review.
-
Alanine aminotransferase predicts incident steatotic liver disease of metabolic etiology: Long life to the old biomarker!World J Gastroenterol. 2024 Jun 28;30(24):3016-3021. doi: 10.3748/wjg.v30.i24.3016. World J Gastroenterol. 2024. PMID: 38983954 Free PMC article.
-
Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions.Gastroenterol Rep (Oxf). 2024 Apr 26;12:goae029. doi: 10.1093/gastro/goae029. eCollection 2024. Gastroenterol Rep (Oxf). 2024. PMID: 38681750 Free PMC article. Review.
References
-
- Schaffner F., Thaler H. Nonalcoholic fatty liver disease. Prog. Liver Dis. 1986;8:283–298. - PubMed
-
- Lonardo A., Bellini M., Tondelli E., Frazzoni M., Grisendi A., Pulvirenti M., Casa G.D. Nonalcoholic steatohepatitis and the “bright liver syndrome”: Should a recently expanded clinical entity be further expanded? Am. J. Gastroenterol. 1995;90:2072–2074. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

