Established and Evolving Roles of the Multifunctional Non-POU Domain-Containing Octamer-Binding Protein (NonO) and Splicing Factor Proline- and Glutamine-Rich (SFPQ)
- PMID: 38248868
- PMCID: PMC10801543
- DOI: 10.3390/jdb12010003
Established and Evolving Roles of the Multifunctional Non-POU Domain-Containing Octamer-Binding Protein (NonO) and Splicing Factor Proline- and Glutamine-Rich (SFPQ)
Abstract
It has been more than three decades since the discovery of multifunctional factors, the Non-POU-Domain-Containing Octamer-Binding Protein, NonO, and the Splicing Factor Proline- and Glutamine-Rich, SFPQ. Some of their functions, including their participation in transcriptional and posttranscriptional regulation as well as their contribution to paraspeckle subnuclear body organization, have been well documented. In this review, we focus on several other established roles of NonO and SFPQ, including their participation in the cell cycle, nonhomologous end-joining (NHEJ), homologous recombination (HR), telomere stability, childhood birth defects and cancer. In each of these contexts, the absence or malfunction of either or both NonO and SFPQ leads to either genome instability, tumor development or mental impairment.
Keywords: ATR/ATM; DNA damage response; NonO/p54nrb; SFPQ/PSF; birth defects; cancer; cell cycle; checkpoint control.
Conflict of interest statement
These authors declare no conflicts of interest.
Figures
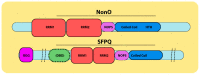
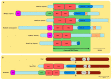

Similar articles
-
Arginine methylation and citrullination of splicing factor proline- and glutamine-rich (SFPQ/PSF) regulates its association with mRNA.RNA. 2015 Mar;21(3):347-59. doi: 10.1261/rna.045138.114. Epub 2015 Jan 20. RNA. 2015. PMID: 25605962 Free PMC article.
-
IGFBP-3 interacts with NONO and SFPQ in PARP-dependent DNA damage repair in triple-negative breast cancer.Cell Mol Life Sci. 2019 May;76(10):2015-2030. doi: 10.1007/s00018-019-03033-4. Epub 2019 Feb 6. Cell Mol Life Sci. 2019. PMID: 30725116 Free PMC article.
-
Loss of NPPA-AS1 promotes heart regeneration by stabilizing SFPQ-NONO heteromer-induced DNA repair.Basic Res Cardiol. 2022 Mar 5;117(1):10. doi: 10.1007/s00395-022-00921-y. Basic Res Cardiol. 2022. PMID: 35247074
-
Involvement of paraspeckle components in viral infections.Nucleus. 2024 Dec;15(1):2350178. doi: 10.1080/19491034.2024.2350178. Epub 2024 May 8. Nucleus. 2024. PMID: 38717150 Free PMC article. Review.
-
[SFPQ and NONO Proteins and Long Non-Coding NEAT1 RNA: Cellular Functions and Role in the HIV-1 Life Cycle].Mol Biol (Mosk). 2022 Mar-Apr;56(2):259-274. doi: 10.31857/S0026898422020161. Mol Biol (Mosk). 2022. PMID: 35403619 Review. Russian.
Cited by
-
Virus-modified paraspeckle-like condensates are hubs for viral RNA processing and their formation drives genomic instability.Nat Commun. 2024 Nov 26;15(1):10240. doi: 10.1038/s41467-024-54592-5. Nat Commun. 2024. PMID: 39592606 Free PMC article.
-
Interrogation of mirror-image l-RNA-protein interactions reveals key mechanisms of single-stranded G-rich l-RNA cytotoxicity and a potential mitigation strategy.Chem Sci. 2025 Mar 20;16(17):7560-7572. doi: 10.1039/d5sc00596e. eCollection 2025 Apr 30. Chem Sci. 2025. PMID: 40171033 Free PMC article.
-
An N-terminal and ankyrin repeat domain interactome of Shank3 identifies the protein complex with the splicing regulator Nono in mice.Genes Cells. 2024 Sep;29(9):746-756. doi: 10.1111/gtc.13142. Epub 2024 Jul 4. Genes Cells. 2024. PMID: 38964745 Free PMC article.
-
Exploring Molecular Drivers of PARPi Resistance in BRCA1-Deficient Ovarian Cancer: The Role of LY6E and Immunomodulation.Int J Mol Sci. 2024 Sep 27;25(19):10427. doi: 10.3390/ijms251910427. Int J Mol Sci. 2024. PMID: 39408764 Free PMC article.
-
LncRNA-536 and RNA Binding Protein RBM25 Interactions in Pulmonary Arterial Hypertension.bioRxiv [Preprint]. 2024 Aug 28:2024.08.27.610011. doi: 10.1101/2024.08.27.610011. bioRxiv. 2024. Update in: Arterioscler Thromb Vasc Biol. 2025 Aug;45(8):e374-e391. doi: 10.1161/ATVBAHA.125.322734. PMID: 39253448 Free PMC article. Updated. Preprint.
References
-
- Dong B., Horowitz D.S., Kobayashi R., Krainer A.R. Purification and cDNA Cloning of HeLa Cell P54nrb, a Nuclear Protein with Two RNA Recognition Motifs and Extensive Homology to Human Splicing Factor PSF and Drosophila NONA/BJ6. Nucleic Acids Res. 1993;21:4085–4092. doi: 10.1093/nar/21.17.4085. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

