Cardiovascular Computed Tomography in the Diagnosis of Cardiovascular Disease: Beyond Lumen Assessment
- PMID: 38248892
- PMCID: PMC10816599
- DOI: 10.3390/jcdd11010022
Cardiovascular Computed Tomography in the Diagnosis of Cardiovascular Disease: Beyond Lumen Assessment
Abstract
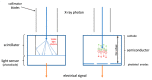
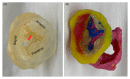
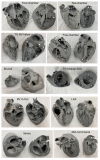
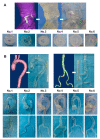
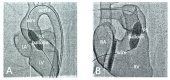
Cardiovascular CT is being widely used in the diagnosis of cardiovascular disease due to the rapid technological advancements in CT scanning techniques. These advancements include the development of multi-slice CT, from early generation to the latest models, which has the capability of acquiring images with high spatial and temporal resolution. The recent emergence of photon-counting CT has further enhanced CT performance in clinical applications, providing improved spatial and contrast resolution. CT-derived fractional flow reserve is superior to standard CT-based anatomical assessment for the detection of lesion-specific myocardial ischemia. CT-derived 3D-printed patient-specific models are also superior to standard CT, offering advantages in terms of educational value, surgical planning, and the simulation of cardiovascular disease treatment, as well as enhancing doctor-patient communication. Three-dimensional visualization tools including virtual reality, augmented reality, and mixed reality are further advancing the clinical value of cardiovascular CT in cardiovascular disease. With the widespread use of artificial intelligence, machine learning, and deep learning in cardiovascular disease, the diagnostic performance of cardiovascular CT has significantly improved, with promising results being presented in terms of both disease diagnosis and prediction. This review article provides an overview of the applications of cardiovascular CT, covering its performance from the perspective of its diagnostic value based on traditional lumen assessment to the identification of vulnerable lesions for the prediction of disease outcomes with the use of these advanced technologies. The limitations and future prospects of these technologies are also discussed.
Keywords: 3D; 3D printing; artificial intelligence; cardiac computed tomography; coronary artery disease; diagnosis; mixed reality; virtual reality; visualization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











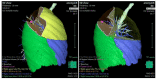
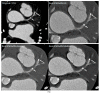

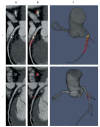
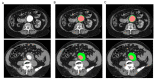
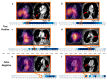
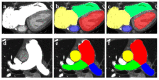
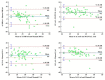


Similar articles
-
Cardiovascular computed tomography in cardiovascular disease: An overview of its applications from diagnosis to prediction.J Geriatr Cardiol. 2024 May 28;21(5):550-576. doi: 10.26599/1671-5411.2024.05.002. J Geriatr Cardiol. 2024. PMID: 38948894 Free PMC article.
-
Synchrotron radiation computed tomography assessment of calcified plaques and coronary stenosis with different slice thicknesses and beam energies on 3D printed coronary models.Quant Imaging Med Surg. 2019 Jan;9(1):6-22. doi: 10.21037/qims.2018.09.11. Quant Imaging Med Surg. 2019. PMID: 30788242 Free PMC article.
-
Clinical Applications of Mixed Reality and 3D Printing in Congenital Heart Disease.Biomolecules. 2022 Oct 24;12(11):1548. doi: 10.3390/biom12111548. Biomolecules. 2022. PMID: 36358899 Free PMC article.
-
Clinical Applications and Advancements of Positron Emission Tomography/Computed Tomography in Cardio-Oncology: A Comprehensive Literature Review and Emerging Perspectives.Curr Oncol Rep. 2024 Nov;26(11):1442-1451. doi: 10.1007/s11912-024-01598-3. Epub 2024 Sep 25. Curr Oncol Rep. 2024. PMID: 39320577 Review.
-
Three-dimensional printing, holograms, computational modelling, and artificial intelligence for adult congenital heart disease care: an exciting future.Eur Heart J. 2022 Jul 21;43(28):2672-2684. doi: 10.1093/eurheartj/ehac266. Eur Heart J. 2022. PMID: 35608227 Review.
Cited by
-
Current Challenges in Diagnosis and Treatment of Cardiovascular Disease.J Pers Med. 2024 Jul 25;14(8):786. doi: 10.3390/jpm14080786. J Pers Med. 2024. PMID: 39201978 Free PMC article.
-
Cardiovascular computed tomography in cardiovascular disease: An overview of its applications from diagnosis to prediction.J Geriatr Cardiol. 2024 May 28;21(5):550-576. doi: 10.26599/1671-5411.2024.05.002. J Geriatr Cardiol. 2024. PMID: 38948894 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

